 |
| 1 |  | 
Which expression for Ka corresponds to the equilibrium below in dilute solution?
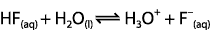 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 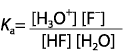 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | D) | 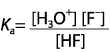 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q1d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|
|
 |
| 2 |  | 
The percent dissociation of an acid is affected by |
|  | A) | the initial concentration of the weak acid only |
|  | B) | the Ka value for the acid and the initial concentration of the weak acid |
|  | C) | the Ka value for the acid only |
|  | D) | the final concentration of the weak acid only |
|
|
 |
| 3 |  | 
Under what circumstances can the amount of acid that dissociates be considered negligible, thus simplifying the mathematics? |
|  | A) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q3c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | D) | Never |
|
|
 |
| 4 |  | 
A solution of a weak acid is prepared in which the equilibrium concentrations of HA and A– are equal. The Ka of the acid is 1.3 x 10–6. What is the pH of the solution? |
|  | A) | 5.89 |
|  | B) | 1.3 x 10–6 |
|  | C) | 8.11 |
|  | D) | There is not enough information to answer the question. |
|
|
 |
| 5 |  | 
Which of the following statements is true? |
|  | A) | Nitrogen-containing compounds are Arrhenius bases and produce OH– in water. |
|  | B) | Nitrogen-containing compounds are Bronsted-Lowry bases that donate a proton in water. |
|  | C) | Nitrogen-containing compounds are Bronsted-Lowry bases that accept a proton in water. |
|  | D) | Nitrogen-containing compounds are Arrhenius acids which produce protons in water. |
|
|
 |
| 6 |  | 
Given that Py represents pyridine, a nitrogen-containing weak base, which of the following is the correct expression for Kb of a dilute solution of Py?
|
|  | A) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6a_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6a_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | B) | 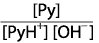 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6b_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6b_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6c_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6c_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6d_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128793/atlCHEMISTRY_CH15_QUIZ2_Q6d_rev.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|
 |
| 7 |  | 
The Kb values for several bases are shown. Which value represents the strongest base? |
|  | A) | Kb = 2.9 x 10–4 |
|  | B) | Kb = 4.5 x 10–7 |
|  | C) | Kb = 6.2 x 10–8 |
|  | D) | Kb = 3.2 x 10–12 |
|
|
 |
| 8 |  | 
Which of the following combinations of solutes would result in a buffer solution? |
|  | A) | Ammonia and hydrochloric acid |
|  | B) | Acetic acid and sodium hydroxide |
|  | C) | Ammonia and ammonium chloride |
|  | D) | Ammonium chloride and sodium acetate |
|
|
 |
| 9 |  | 
Which of the following statements is true regarding a buffer solution? |
|  | A) | Buffer capacity depends on the initial pH of the solution. |
|  | B) | Buffer capacity depends on the ratio of the concentrations of the conjugate acid/base pair in solution. |
|  | C) | A concentrated buffer solution is less resistant to pH changes than a dilute one. |
|  | D) | If the ratio of the concentrations of the conjugate acid/base pair in solution is close to 1, the buffer capacity is at a minimum. |
|
|
 |
| 10 |  | 
Which statement is not true regarding buffers in the blood? |
|  | A) | The most important buffer system is the HCO3–CO3– pair. |
|  | B) | Because HCO3– is amphoteric it can react with both hydronium and hydroxide ions in the blood. |
|  | C) | The pH of human blood is maintained between 7.0 and 7.5 by buffers. |
|  | D) | Sodium bicarbonate must be ingested to produce the bicarbonate necessary for the blood buffer system. |
|
|

