 |
1 |  | 
This is the Lewis structure for ethanol, C2H5OH. Calculate the energy change associated with breaking all of the bonds in ethanol.
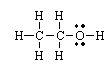 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 1574 kJ |
|  | B) | 2772 kJ |
|  | C) | 2883 kJ |
|  | D) | 3239 kJ |
 |
 |
2 |  | 
Which equation represents the process of "cat cracking," producing more useful molecular weight fractions from crude oil? |
|  | A) | C16H34 + catalyst ----> C8H18 + C8H16 |
|  | B) | 2 C2H6 + 7 O2 + catalyst ----> 4 CO2 + 6 H2O |
|  | C) | C4H10 + C3H6 + catalyst ----> C7H16 |
|  | D) | CH2CH2 + H2O + catalyst ----> CH3CH2OH |
 |
 |
3 |  | 
Modern fossil fuel power plants do not operate anywhere near 100% efficiency. Why not? |
|  | A) | Although 100% efficiency is possible, it is too expensive to design the plant to operate with this high of an efficiency. |
|  | B) | 100% efficiency is impossible. Organized energy is always being transformed into random motion, limiting efficiency. |
|  | C) | Although 100% efficiency is possible, the Law of Conservation of Energy governs the conversion of energy from one form to another. |
|  | D) | 100% efficiency is impossible. One of the driving forces for any chemical reaction is the release of heat energy. |
 |
 |
4 |  | 
Which structure shows the same structural isomer as the isomer of pentane shown here? (All hydrogen atoms have been omitted for clarity.)
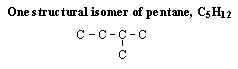 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 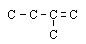 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) | 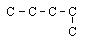 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 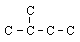 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) | 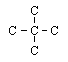 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A4_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
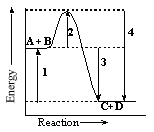
5 |  | 
Which line segment in the diagram represents the net energy change for this reaction?
A + B ----> C + D
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | 1 |
|  | B) | 2 |
|  | C) | 3 |
|  | D) | 4 |
 |
 |
6 |  | 
How much energy will be required to break the bonds between nitrogen atoms in one mole of nitrogen gas, N2? |
|  | A) | 160 kJ |
|  | B) | 428 kJ |
|  | C) | 578 kJ |
|  | D) | 946 kJ |
 |
 |
7 |  | 
Which balanced chemical equation represents a process of complete combustion of a hydrocarbon? |
|  | A) | 2 C8H18(l) + 17 O2(g) ----> 16 CO (g) + 18 H2O (g) + heat |
|  | B) | C2H4(g) + 3 O2(g) ----> 2 CO2(g) + 2 H2O (g) + heat |
|  | C) | C6H12O6(s) ----> 2 C2H5OH (l) + 2 CO2(g) + heat |
|  | D) | C2H4(g) + H2(g) ----> C2H6(g) + heat |
 |
 |
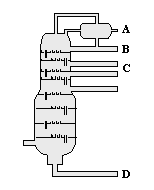
8 |  | 
At which location in this distillation column will petroleum gas, C1 - C4 hydrocarbons, be most likely to be collected?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | A |
|  | B) | B |
|  | C) | C |
|  | D) | D |
 |
 |
9 |  | 
Which statement is correct at the present time for total oil used in the U.S.? |
|  | A) | More than 50% of the oil used in the U.S. is from domestic production. |
|  | B) | More than 50% of the oil used in the U.S. is imported. |
|  | C) | More than 50% of the oil used in the U.S. is from the Alaskan North Slope. |
|  | D) | More than 50% of the oil used in the U.S. is from other sources, such as natural gas liquids. |
 |
 |
10 |  | 
Statement: Energy is neither created nor destroyed in a chemical reaction. This statement of the Law of Conservation of Energy is also known as the |
|  | A) | First Law of Thermodynamics. |
|  | B) | Second Law of Thermodynamics. |
|  | C) | Third Law of Thermodynamics. |
|  | D) | driving force for a chemical reaction. |
 |
 |
11 |  | 
How many kJ of food energy do you get from eating a serving of a snack labeled as providing 150 Cal per serving? |
|  | A) | 3.6 x 101 kJ |
|  | B) | 1.5 x 102 kJ |
|  | C) | 2.8 x 102 kJ |
|  | D) | 6.3 x 102 kJ |
 |
 |
12 |  | 
Which is a correct statement about fossil fuels? |
|  | A) | Fossil fuels are produced by large farming operations, such as growing soybeans or corn. They are renewable resources. |
|  | B) | Fossil fuels are carbon-containing skeletons from animals, which have undergone decay under high temperatures and pressure. They are renewable resources. |
|  | C) | Fossil fuels are gaseous carbon-based fuels such as carbon monoxide, natural gas, and carbon dioxide. They are renewable resources. |
|  | D) | Fossil fuels originated as plant or animal matter 150-300 million years ago. They are nonrenewable resources. |
 |
 |
13 |  | 
Which statement is correct? |
|  | A) | Forming bonds always requires energy. It is an exothermic process. |
|  | B) | Forming bonds always requires energy. It is an endothermic process. |
|  | C) | Forming bonds always releases energy. It is an endothermic process. |
|  | D) | Forming bonds always releases energy. It is an exothermic process. |
 |
 |
14 |  | 
Which graph shows an exothermic reaction with curve A being the catalyzed reaction and curve B being the uncatalyzed reaction? |
|  | A) | 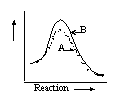 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) | 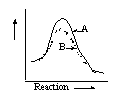 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 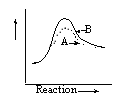 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) | 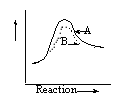 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51618/4A14_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
15 |  | 
Consider the single bond energy values: H-H = 436 kJ/mol; C-H = 416 kJ/mol; N-H = 391 kJ/mol; O-H = 467 kJ/mol. Which lists the bonds in the order of weakest bond to strongest bond? |
|  | A) | H-H, H-C, H-N |
|  | B) | H-H, H-C, H-O |
|  | C) | H-C, H-H, H-O |
|  | D) | H-N, H-H, H-C |
 |

