The Organic Chemistry Test consists of 20 questions. Correct answers will be provided after your answers have been submitted. If they are available, possible solutions can be accessed by clicking an additional link at the bottom of the answer, which will open a problem solution screen. If you bring up such a solution screen be sure to close it by clicking the button in the upper left corner or upper right corner.
 |
1 |  | 
Organic chemistry is the study of |
|  | A) | any compound from any living thing. |
|  | B) | carbon-containing compounds that were formed by living things. |
|  | C) | any compound with carbon as the principal element. |
|  | D) | none of the above. |
 |
 |
2 |  | 
Carbon can form many different compounds because it can |
|  | A) | make a molecule in the shape of a cube, tetrahedron, or cylinder. |
|  | B) | combine with other carbon atoms in addition to other elements. |
|  | C) | combine with more metals than other elements. |
|  | D) | combine with more nonmetals than other elements. |
 |
 |
3 |  | 
A hydrocarbon is an organic compound consisting of |
|  | A) | water and carbon. |
|  | B) | any number of elements as long as they include carbon and hydrogen. |
|  | C) | carbon and hydrogen. |
|  | D) | carbon, hydrogen, and oxygen. |
 |
 |
4 |  | 
A hydrocarbon with two double covalent bonds between carbon atoms is a (an) |
|  | A) | alkane. |
|  | B) | alkene. |
|  | C) | alkyne. |
|  | D) | aromatic hydrocarbon. |
 |
 |
5 |  | 
Organic compounds called isomers have |
|  | A) | the same molecular formulas but different physical structures. |
|  | B) | different molecular formulas with the same physical structures. |
|  | C) | different molecular formulas with the same chemical properties. |
|  | D) | none of the above. |
 |
 |
6 |  | 
The number of carbon atoms in a molecule of octane is |
|  | A) | 1 |
|  | B) | 2 |
|  | C) | 4 |
|  | D) | 8 |
 |
 |
7 |  | 
Organic compounds called aromatic hydrocarbons are compounds that |
|  | A) | have a wonderful odor. |
|  | B) | are based on the benzene ring structure. |
|  | C) | occur in nature with all carbons bonds saturated. |
|  | D) | occur in nature with all carbon bonds unsaturated. |
 |
 |
8 |  | 
A gasoline mixture of hydrocarbons that burns very rapidly has |
|  | A) | a low octane number. |
|  | B) | a high octane number. |
|  | C) | many branched chains. |
|  | D) | smaller hydrocarbon molecules. |
 |
 |
9 |  | 
An organic molecule with a general formula of ROH is a (an) |
|  | A) | ether. |
|  | B) | ester. |
|  | C) | alcohol. |
|  | D) | organic acid. |
 |
 |
10 |  | 
An organic molecule with a general formula of RCOOH is a (an) |
|  | A) | ester. |
|  | B) | organic acid. |
|  | C) | ketone. |
|  | D) | aldehyde. |
 |
 |
11 |  | 
The characteristic odor and taste of fruit such as bananas, oranges, and pineapples comes from certain |
|  | A) | ketones. |
|  | B) | ethers. |
|  | C) | aldehydes. |
|  | D) | esters. |
 |
 |
12 |  | 
The human body breaks down starches to |
|  | A) | monosaccharides. |
|  | B) | simple sugars. |
|  | C) | glucose. |
|  | D) | any of the above. |
 |
 |
13 |  | 
The direct reserve source of energy in the muscles of a human is |
|  | A) | glycol. |
|  | B) | glycerol. |
|  | C) | glycogen. |
|  | D) | dextrose. |
 |
 |
14 |  | 
All proteins are made up of a side chain and |
|  | A) | alpha-amino acid. |
|  | B) | amine. |
|  | C) | a nitrogen atom. |
|  | D) | peptide. |
 |
 |
15 |  | 
Fats and oils are esters formed from three fatty acids and glycerol into a (an) |
|  | A) | alpha-amino acid. |
|  | B) | polysaccharide. |
|  | C) | triglyceride. |
|  | D) | disaccharide. |
 |
 |
16 |  | 
Which of the following is a polymer? |
|  | A) | Cellulose. |
|  | B) | Polyethylene. |
|  | C) | Wool. |
|  | D) | All are polymers. |
 |
 |
17 |  | 
The IUPAC name for the molecule below is
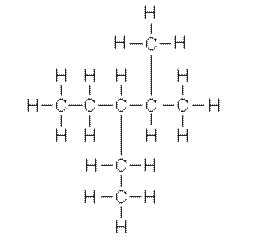 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121104/chap12_image1.jpg','popWin', 'width=304,height=311,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (7.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121104/chap12_image1.jpg','popWin', 'width=304,height=311,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (7.0K)</a> |
|  | A) | octane. |
|  | B) | 3-ethyl-4-methylpentane. |
|  | C) | methylheptane. |
|  | D) | 3-ethyl-2-methylpentane. |
 |
 |
18 |  | 
An alkane with 3 carbon atoms would have how many hydrogen atoms in the molecule? |
|  | A) | 4 |
|  | B) | 6 |
|  | C) | 8 |
|  | D) | 10 |
 |
 |
19 |  | 
The R in R-COOH or R-C=O stands for |
|  | A) | a reactive atom. |
|  | B) | a separate functional group. |
|  | C) | any hydrocarbon group. |
|  | D) | the right side of the molecule. |
 |
 |
20 |  | 
When wine "goes bad," the ethanol is converted into |
|  | A) | CH3COOH |
|  | B) | CH3 OCH3 |
|  | C) | CH3CH2OH |
|  | D) | CH3OH |
 |

