 |
1 |  | 
An electron with a mass of 9.11 × 10-31 kg has a velocity of 4.3 × 106 m/s in the innermost orbit of a hydrogen atom. What is the de Broglie wavelength of the electron? |
|  | |
 |
 |
2 |  | 
Calculate the energy (a) in eV and (b) in joules of the third energy level (n = 3) of a hydrogen atom. |
|  | |
 |
 |
3 |  | 
How much energy is needed to move an electron in a hydrogen atom from the ground state (n = 1) to n = 3? Give the answer (a) in joules and (b) in eV. |
|  | |
 |
 |
4 |  | 
What frequency of light is emitted when an electron in a hydrogen atom jumps from n = 2 to the ground state (n = 1)? |
|  | |
 |
 |
5 |  | 
How much energy is needed to completely remove an electron from n = 2 in a hydrogen atom? |
|  | |
 |
 |
6 |  | 
If the charge-to-mass ratio of a proton is 9.58 × 107 coulomb/kilogram and the charge is 1.60 × 10-19 coulomb, what is the mass of the proton? |
|  | |
 |
 |
7 |  | 
An electron wave making a standing wave in a hydrogen atom has a wavelength of 8.33 × 10-11 m. If the mass of the electron is 9.11 × 10-31 kg, what is the velocity of the electron according to the de Broglie equation? |
|  | |
 |
 |
8 |  | 
Using any reference you wish, write the complete electron configurations for (a) nitrogen, (b) phosphorus, and (c) chlorine. |
|  | |
 |
 |
9 |  | 
Explain how you know that you have the correct total number of electrons in your answers for 8a, 8b, and 8c. |
|  | |
 |
 |
10 |  | 
Referring to Figure 8.18 only, write the complete electron configuration for (a) neon, (b) sulfur, and (c) calcium. |
|  | |
 |
 |
11 |  | 
Boron has two naturally occurring isotopes, boron-10 and boron-11. Boron-10 has a mass of 10.0129 relative to carbon-12 and makes up 19.78 percent of all naturally occurring boron. Boron-11 has a mass of 11.00931 compared to carbon-12 and makes up the remaining 80.22 percent. What is the atomic weight of boron? |
|  | |
 |
 |
12 |  | 
Identify the number of protons, neutrons, and electrons in the following isotopes:
| (a) | 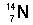 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_1.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_1.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | | (b) | 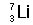 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_2.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_2.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | | (c) | 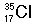 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_3.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_3.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | | (d) | 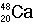 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_4.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_4.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | | (e) | 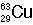 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_5.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_5.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> | | (f) | 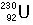 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_6.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072509783/121100/chap08parexb_6.jpg','popWin', 'width=83,height=95,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | |
 |
 |
13 |  | 
Identify the period and the family in the periodic table for the following elements:
(a) Xenon
(b) Potassium
(c) Chromium
(d) Argon
(e) Bromine
(f) Barium |
|  | |
 |
 |
14 |  | 
How many outer-orbital electrons are found in an atom of
(a) Na
(b) P
(c) Br
(d) I
(e) Te
(f) Sr |
|  | |
 |
 |
15 |  | 
Write electron dot notations for the following elements:
(a) Aluminum
(b) Fluorine
(c) Magnesium
(d) Sodium
(e) Carbon
(f) Chlorine |
|  | |
 |
 |
16 |  | 
Identify the charge on the following ions:
(a) Aluminum
(b) Chlorine
(c) Magnesium
(d) Sodium
(e) Sulfur
(f) Hydrogen |
|  | |
 |
 |
17 |  | 
Use the periodic table to identify if the following are metals, nonmetals, or semiconductors:
(a) Radon
(b) Francium
(c) Arsenic
(d) Phosphorus
(e) Hafnium
(f) Uranium |
|  | |
 |
 |
18 |  | 
From their charges, predict the periodic table family number for the following ions:
(a) F-1
(b) Li+1
(c) B+3
(d) O-2
(e) Be+2
(f) Si+4 |
|  | |
 |
 |
19 |  | 
Use chemical symbols and numbers to identify the following isotopes:
(a) Potassium-39
(b) Neon-22
(c) Tungsten-184
(d) Iodine-127 |
|  | |
 |

