 |
1 |  | 
The most important force in Chemistry is? |
|  | A) | Nuclear |
|  | B) | Electrical |
|  | C) | Gravitational |
 |
 |
2 |  | 
The theory which describes the formation of a bond by the overlap of half-filled atomic orbitals is? |
|  | A) | VSEPR Theory |
|  | B) | Molecular Orbital Theory |
|  | C) | Valence Bond Theory |
 |
 |
3 |  | 
A sigma (s) bond is characterized by a high electron density between the atoms, and is cylindrically symmetric about the internuclear axis. |
|  | A) | True |
|  | B) | False |
 |
 |
4 |  | 
Hybridization is? |
|  | A) | generated by "mixing" s and p orbitals |
|  | B) | an attempt to explain the bonding observed in organic molecules |
|  | C) | an attempt to explain the geometry observed in organic molecules |
|  | D) | A and B |
|  | E) | A and C |
|  | F) | all of the above. |
 |
 |
5 |  | 
A methylene group is? |
|  | A) | A CH4 group |
|  | B) | A CH3 group |
|  | C) | A CH2 group |
|  | D) | A CH group |
 |
 |
6 |  | 
A sec-butyl group is? |
|  | A) | A (CH3)4C group |
|  | B) | A (CH3)3C- group |
|  | C) | A (CH3)2CHCH2- group |
|  | D) | A CH3CH2CH(CH3)- group |
|  | E) | A CH3(CH2)3- group |
 |
 |
7 |  | 
Hydrocarbons are found in petroleum, which is a mixture of numerous different types of alkanes, alkenes, and arenes. They are separated by distillation. Which other processes are important in making petroleum more useful? |
|  | A) | Cracking |
|  | B) | Reforming |
|  | C) | Decomposing |
|  | D) | A and B |
|  | E) | A and C |
 |
 |
8 |  | 
The major intermolecular force in alkanes is? |
|  | A) | Dipole-dipole forces |
|  | B) | Dipole-induced dipole forces |
|  | C) | Induced dipole-induced dipole forces |
|  | D) | Hydrogen bonding |
 |
 |
9 |  | 
Arrange the following compounds according
to the strength of the intermolecular forces.
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_9.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_9.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (15.0K)</a> |
|  | A) | 1 > 2 > 3 |
|  | B) | 1 > 3 > 2 |
|  | C) | 2 > 3 > 1 |
|  | D) | 2 > 1 > 3 |
|  | E) | 3 > 1 > 2 |
 |
 |
10 |  | 
The relative stability of a homologous series of alkanes depends on? |
|  | A) | Intermolecular forces |
|  | B) | Intramolecular forces |
|  | C) | The number of carbon atoms in the molecule |
|  | D) | The molecular weight |
|  | E) | none of the above |
 |
 |
11 |  | 
Arrange the following compounds in order of decreasing oxidation state
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/211137/2_11.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/211137/2_11.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> |
|  | A) | 1 > 2 > 3 |
|  | B) | 1 > 3 > 2 |
|  | C) | 2 > 3 > 1 |
|  | D) | 2 > 1 > 3 |
|  | E) | 3 > 1 > 2 |
|  | F) | 3 > 2 > 1 |
 |
 |
12 |  | 
The carbon atoms in benzene are?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_12.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_12.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (11.0K)</a> |
|  | A) | sp4 hybridiz |
|  | B) | sp3 hybridiz |
|  | C) | sp2 hybridiz |
|  | D) | sp hybridiz |
 |
 |
13 |  | 
The central carbon atom has what type of hybridization?
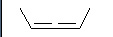 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_13.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0072828374/337328/2_13.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> |
|  | A) | sp4 hybridiz |
|  | B) | sp3 hybridiz |
|  | C) | sp2 hybridiz |
|  | D) | sp hybridiz |
 |

