 |
| 1 |  | 
Which of the following pairs contains one polar bond and one non-polar bond? |
|  | A) | C-C and O-O |
|  | B) | C-O and C-C |
|  | C) | O-C and N-H |
|  | D) | F-F and C-Br |
|
|
 |
| 2 |  | 
Which of the following is true for any polar molecule? |
|  | A) | It has identical polar bonds acting in opposite directions. |
|  | B) | It has equal dipoles acting in opposite directions. |
|  | C) | It contains at least one bond dipole that is not counteracted by another. |
|  | D) | It contains no polar bonds. |
|
|
 |
| 3 |  | 
Which of the following compounds is polar? |
|  | A) | CO2 |
|  | B) | CCl4 |
|  | C) | H2O |
|  | D) | CH3CH2CH3 |
|
|
 |
| 4 |  | 
Which of the following statements is not true? |
|  | A) | Molecules with functional groups are less reactive than those without them. |
|  | B) | Two molecules with the same functional group will probably have similar chemical properties. |
|  | C) | Two molecules with the same functional group will probably have similar physical properties. |
|  | D) | The identity of the functional group in a molecule can be deduced from its name. |
|
|
 |
| 5 |  | 
In which of the following are the intermolecular forces arranged in order of increasing strength? |
|  | A) | Hydrogen bonding, dipole-dipole forces and dispersion forces |
|  | B) | Dispersion forces, hydrogen bonding, and dipole-dipole forces |
|  | C) | Dispersion forces, dipole-dipole forces and hydrogen bonding |
|  | D) | Dipole-dipole forces, hydrogen bonding and dispersion forces |
|
|
 |
| 6 |  | 
In which of the following are the compounds arranged in order of increasing boiling point? |
|  | A) | Butane, hexane, methanol |
|  | B) | Methanol, butane, hexane |
|  | C) | Hexane, butane, methanol |
|  | D) | Butane, methanol, hexane |
|
|
 |
| 7 |  | 
In which of the following are the compounds arranged in order of decreasing solubility in water? |
|  | A) | CH3CH2OH, C6H6, CH3CH2 CH2 CH2 CH2OH |
|  | B) | C6H6, CH3CH2 CH2 CH2 CH2OH, CH3CH2OH |
|  | C) | CH3CH2 CH2 CH2 CH2OH, C6H6, CH3CH2OH |
|  | D) | CH3CH2OH, CH3CH2 CH2 CH2 CH2OH, C6H6 |
|
|
 |
| 8 |  | 
Classify the following reaction.
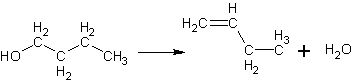 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128788/atlCHEMISTRY_CH10_QUIZ1_Q8.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (4.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128788/atlCHEMISTRY_CH10_QUIZ1_Q8.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (4.0K)</a>
|
|  | A) | Addition |
|  | B) | Elimination |
|  | C) | Oxidation |
|  | D) | Substitution |
|
|
 |
| 9 |  | 
Classify the following reaction.
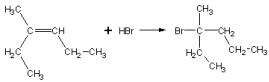 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128788/atlCHEMISTRY_CH10_QUIZ1_Q9.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128788/atlCHEMISTRY_CH10_QUIZ1_Q9.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a>
|
|  | A) | Addition |
|  | B) | Elimination |
|  | C) | Oxidation |
|  | D) | Substitution |
|
|
 |
| 10 |  | 
When two organic molecules combine and one of the products is a water molecule the reaction is an example of |
|  | A) | elimination |
|  | B) | condensation |
|  | C) | hydrolysis |
|  | D) | addition |
|
|

