 |
| 1 |  | 
When does the rate of the forward reaction of a reversible reaction decrease? |
|  | A) | when the temperature is increased |
|  | B) | when the initial concentration of the reactants increases |
|  | C) | when the rate of the reverse reaction increases |
|  | D) | when the rate of the reverse reaction remains constant |
|
|
 |
| 2 |  | 
What is the equilibrium expression for the following reaction?
4NH3(g) + 5O2(g) ⇔ 4NO(g) + 6H2O(g)
|
|  | A) | 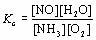 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2a.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2a.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | B) | 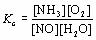 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2b.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2b.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | C) | 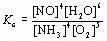 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2c.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2c.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a>
|
|  | D) | 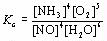 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2d.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q2d.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a>
|
|
|
 |
| 3 |  | 
What is the reaction equation that corresponds to the following equilibrium expression?
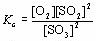 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q3.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070938539/128791/ATLCHEMISTRY_CH13_QUIZ2_Q3.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | A) | 2SO3(g) ⇔ 2O2 + 2SO2(g)
|
|  | B) | 2O2 + 2SO2(g) ⇔ 2SO3(g)
|
|  | C) | O2 + 2SO2(g) ⇔ 2SO3(g)
|
|  | D) | 2SO3(g) ⇔ O2 + 2SO2(g) |
|
|
 |
| 4 |  | 
The following reaction took place at a certain temperature in a 12.5 L flask. If the concentrations at equilibrium are [SO3] = 2.33 mol/L, [O2] = 1.23 mol/L, and [SO2] = 0.433 mol/L, what is the equilibrium constant?
2SO3(g) = O2(g) + 2SO2(g)
|
|  | A) | 0.234 |
|  | B) | 0.229 |
|  | C) | 23.5 |
|  | D) | 0.0425 |
|
|
 |
| 5 |  | 
Which of the following statements is not correct? |
|  | A) | The equilibrium constant can be calculated by multiplying the concentrations of products, and then dividing by the concentrations of reactants. |
|  | B) | The intensity of the colour of a solution is related to the concentration of the ions and the depth of the solution. |
|  | C) | Colour, pH, and pressure are properties that can be measured to help determine the concentrations of substances in reactions at equilibrium. |
|  | D) | The equilibrium constant of a reaction can be calculated without knowing the initial concentrations as long as the final concentrations of the reactants and products are known. |
|
|
 |
| 6 |  | 
In which circumstance are reactants favoured and does the equilibrium of the reaction lie far to the left? |
|  | A) | When the value of Kc is very large |
|  | B) | When the value of Kc is equal to one |
|  | C) | When the value of Kc is very small |
|  | D) | When the value of Kc cannot be calculated |
|
|
 |
| 7 |  | 
Which of the following equilibrium constant values indicates products are in excess in a reaction mixture at equilibrium? |
|  | A) | 1.2 x 10-2 |
|  | B) | 1.2 x 10-5 |
|  | C) | 0 |
|  | D) | 1.2 x 105 |
|
|
 |
| 8 |  | 
When may the change in concentration, x, be ignored to help simplify equilibrium calculations? |
|  | A) | When Kc is small compared to the initial concentration |
|  | B) | When Kc is large compared to the initial concentration |
|  | C) | When the initial concentration of a substance is zero |
|  | D) | Never |
|
|

