 |
| 1 |  | 
According to Hess's law of heat summation, which of the following statements is not true? |
|  | A) | The enthalpy change is independent of the number of intermediate steps. |
|  | B) | The enthalpy change depends on the pathway of the process. |
|  | C) | The enthalpy change is independent of the pathway of the process. |
|  | D) | Both A and C. |
|
|
 |
| 2 |  | 
Which of the following is not true? |
|  | A) | Hess's law is valid because enthalpy is considered to be a state function. |
|  | B) | A state function is a property of a system that is determined only by the current conditions of the system. |
|  | C) | A state function is dependent on the path taken by the system to reach its current conditions. |
|  | D) | All of the above |
|
|
 |
| 3 |  | 
Which of the following is true? |
|  | A) | By definition, the enthalpy of formation of an element in its standard state is zero. |
|  | B) | By definition, the enthalpy of formation of an element in its standard state is 1 kJ/mol. |
|  | C) | Diamond is defined as the standard state of carbon. |
|  | D) | Ozone is defined as the standard state of oxygen. |
|
|
 |
| 4 |  | 
Which is true about the following reaction?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | The reactants have a higher potential energy than the products. |
|  | B) | The chemical system loses energy. |
|  | C) | The products have a higher potential energy than the reactants. |
|  | D) | The surroundings gain energy. |
|
|
 |
| 5 |  | 
Which is true about the following reaction?
C(s) + O2(g) → CO2(g)
∆H° = - 393.5 kJ
|
|  | A) | The enthalpy change is greater than the total enthalpy change of the formation of carbon dioxide via the following process
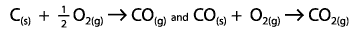 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | B) | The enthalpy change is the same as the total enthalpy change of the formation of carbon dioxide via the following process
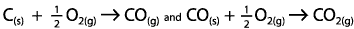 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | C) | The enthalpy change is less than the total enthalpy change of the formation of carbon dioxide via the following process
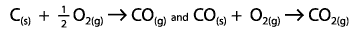 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128795/atlCHEMISTRY_CH17_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | D) | It is not possible. |
|
|
 |
| 6 |  | 
Which of the following is not true? |
|  | A) | The overall enthalpy change of any reaction is the sum of the enthalpy change of the decomposition of the reactants to their elements and the enthalpy change of the formation of the products from their elements. |
|  | B) | In most reactions, the reactants do not break down into their elements and then react to form products. |
|  | C) | The overall enthalpy change of any reaction is the sum of enthalpy change of the decomposition of the products to their elements and the enthalpy change of the formation of the reactants from their elements. |
|  | D) | Using enthalpies of formation to determine the enthalpy of a reaction is consistent with Hess's law. |
|
|
 |
| 7 |  | 
To determine the enthalpy change of a reaction one can |
|  | A) | carry out the reaction in the laboratory |
|  | B) | find reactions that have equations that add up to the target equation and use the enthalpies of reaction of those reactions |
|  | C) | use the standard molar enthalpies of formation for the substances in the equation of the reaction |
|  | D) | All of the above |
|
|
 |
| 8 |  | 
Which of the following is true? |
|  | A) | Bond energy is the specific amount of energy needed to break a bond. |
|  | B) | Breaking bonds is an exothermic process. |
|  | C) | Bond energy is usually measured in mol/kJ. |
|  | D) | Bond formation is an endothermic process. |
|
|

