 |
| 1 |  | 
All atoms of the same element have |
|  | A) | the same number of protons |
|  | B) | the same atomic mass |
|  | C) | the same number of neutrons |
|  | D) | the same mass number |
|
|
 |
| 2 |  | 
What is true about the following atom?
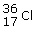 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128780/atlCHEMISTRY_CH02_QUIZ1_Q2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128780/atlCHEMISTRY_CH02_QUIZ1_Q2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | A) | It has 17 protons and 36 neutrons. |
|  | B) | It has 17 protons and 19 electrons. |
|  | C) | It has 36 protons and 17 neutrons. |
|  | D) | It has 17 protons and 19 neutrons. |
|
|
 |
| 3 |  | 
What is another way to reprsent the following isotope?
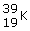 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128780/atlCHEMISTRY_CH02_QUIZ1_Q3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128780/atlCHEMISTRY_CH02_QUIZ1_Q3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | A) | potassium-39 |
|  | B) | potassium-19 |
|  | C) | potassium-20 |
|  | D) | krypton-19 |
|
|
 |
| 4 |  | 
The relative mass of an atom |
|  | A) | is measured in atomic mass units (u) |
|  | B) | is based on the mass of 1 atom of carbon-12 |
|  | C) | is different for different isotopes of an element |
|  | D) | all of the above are true |
|
|
 |
| 5 |  | 
Isotopic abundance |
|  | A) | is the same for each element |
|  | B) | is always expressed as a % |
|  | C) | measures the relative amount of each naturally occurring isotope of an element. |
|  | D) | is always expressed as a decimal fraction. |
|
|
 |
| 6 |  | 
Average atomic mass takes into account |
|  | A) | the mass of each isotope of an element only |
|  | B) | the relative abundance of each isotope of an element only |
|  | C) | the mass and relative abundance of each isotope of an element |
|  | D) | the percentage by mass of each isotope found in nature only |
|
|
 |
| 7 |  | 
Average atomic mass |
|  | A) | is the mass found on the periodic table |
|  | B) | is usually a whole number |
|  | C) | is measured in grams |
|  | D) | equals the mass of the most common isotope |
|
|
 |
| 8 |  | 
When calculating average atomic mass, |
|  | A) | use the abundance expressed as a decimal fraction |
|  | B) | the answer should be closest to the mass of the most abundant isotope |
|  | C) | report the answer with the units of u |
|  | D) | all of the above are true |
|
|
 |
| 9 |  | 
Without doing a calculation, which answer seems the most reasonable for the average atomic mass of boron?
20% boron-10 10.01u
80% boron-11 11.01u |
|  | A) | 11.01u because it is the mass of the most common isotope. |
|  | B) | 10.81u because it is closest but not equal to the mass of the most common isotope. |
|  | C) | 10.51u because it is the average of the masses of the two isotopes. |
|  | D) | 10.01u because it the mass of one of the isotopes. |
|
|

