 |
| 1 |  | 
What is meant by the phrase "the reactants are present in stoichiometric amounts"? |
|  | A) | The reactants are present in equal molar amounts. |
|  | B) | The same mass of each of each reactant is present. |
|  | C) | The reactants are present in the mole ratio that corresponds to the ratio of the coefficients in the balanced chemical equation. |
|  | D) | The reactants are present in the mass ratio that corresponds to the ratio of the coefficients in the balanced chemical equation. |
|
|
 |
| 2 |  | 
In which case are the reactants present in the stoichiometric amounts for the following reaction?
2 N2(g) + 3 H2(g) → 2 NH3(g) |
|  | A) | 0.4 mol of N2 and 0.6 mol of H2 |
|  | B) | 6 g of N2 and 9 g of H2 |
|  | C) | 5 mol of N2 and 5 mol of H2 |
|  | D) | 2 g of N2 and 3 g of H2 |
|
|
 |
| 3 |  | 
The limiting reactant in a chemical reaction |
|  | A) | is the reactant present in the least amount by mass |
|  | B) | determines the amount of product formed |
|  | C) | is the reactant present in the least molar amount |
|  | D) | is left over at the end of the reaction |
|
|
 |
| 4 |  | 
Acetylene (C2H2) is burned with oxygen in a blow torch according to the equation
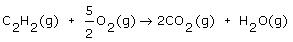 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128782/atlCHEMISTRY_CH04_QUIZ2_Q4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128782/atlCHEMISTRY_CH04_QUIZ2_Q4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
If there is 0.100 mol of acetylene and 0.300 mol of oxygen, which reactant is limiting? |
|  | A) | C2H2 |
|  | B) | O2 |
|  | C) | CO2 |
|  | D) | H2O |
|
|
 |
| 5 |  | 
Ethane (C2H6), an important component of natural gas, burns according to the following balanced equation:
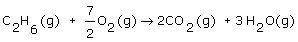 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128782/atlCHEMISTRY_CH04_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128782/atlCHEMISTRY_CH04_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
If there is 0.100 mol of ethane and 0.300 mol of oxygen, which reactant is limiting? |
|  | A) | C2H2 |
|  | B) | O2 |
|  | C) | CO2 |
|  | D) | H2O |
|
|
 |
| 6 |  | 
When performing a stoichiometric calculation, how do you know when you need to identify the limiting reagent? |
|  | A) | You are given information regarding the first reactant. |
|  | B) | You are given information regarding the second reactant. |
|  | C) | You are given information regarding a product formed. |
|  | D) | You are given information regarding two or more reactants. |
|
|
 |
| 7 |  | 
When performing a stoichiometric calculation to determine the amount of CO2 produced by the combustion in air of 4kg of methane, what do you assume? |
|  | A) | There is also 4 kg of oxygen present. |
|  | B) | The question is incomplete. More information is required to do the calculation. |
|  | C) | The amount of oxygen present is not mentioned therefore it is not the limiting reactant. |
|  | D) | The amount of oxygen present is not mentioned therefore it is the limiting reactant. |
|
|
 |
| 8 |  | 
What mass of solid AgCl is produced when 0.200 mol of Ag+ ions are mixed with 0.300 mol of Cl- ions in the following reaction (the molar mass of AgCl is 143.32 g/mol) Ag+(aq) + Cl-(aq) → AgCl(s) |
|  | A) | 71.7 g |
|  | B) | 35.8 g |
|  | C) | 43.0 g |
|  | D) | 28.7 g |
|
|
 |
| 9 |  | 
What mass of solid Ag2S is produced when 0.200 mol of Ag+ ions is mixed with 0.300 mol of S2-ions in the following reaction (the molar mass Ag2S is 247.81 g/mol): Ag+(aq) + S2-(aq) → Ag2S(s) |
|  | A) | 24.8 g |
|  | B) | 37.2 g |
|  | C) | 49.6 g |
|  | D) | 74.3 g |
|
|

