 |
1 |  | 
Atoms are made of |
|  | A) | protons only. |
|  | B) | neutrons only. |
|  | C) | electrons only. |
|  | D) | a, b and c |
|  | E) | a and b only |
 |
 |
2 |  | 
Which statement is false? |
|  | A) | Protons and neutrons balance each other electrically. |
|  | B) | Protons are positively charged. |
|  | C) | Neutrons are neutral. |
|  | D) | Electrons are negatively charged. |
 |
 |
3 |  | 
A positron is |
|  | A) | a particle which has the same mass as an electron but has a positive charge and is short-lived. |
|  | B) | a particle which has the same mass as an electron but has a negative charge and is short-lived. |
|  | C) | a particle which has the same mass as a proton but has a negative charge and is short-lived. |
|  | D) | a particle which has the same mass as an electron but has a neutral charge and is short-lived. |
 |
 |
4 |  | 
Each of the following is a true statement about an AMU except: |
|  | A) | AMU stands for "atomic mass unit". |
|  | B) | There are 6.02 x 10 23 AMU's in one gram. |
|  | C) | "Dalton" is another name for AMU. |
|  | D) | Each proton, neutron and electron has a mass of one AMU |
 |
 |
5 |  | 
Atoms of the same element with different numbers of neutrons are known as |
|  | A) | isotopes. |
|  | B) | ions. |
|  | C) | quarks. |
|  | D) | molecules. |
 |
 |
6 |  | 
Every atom of the same element has |
|  | A) | the same mass. |
|  | B) | the same atomic number. |
|  | C) | the same number of neutrons. |
|  | D) | the same weight. |
 |
 |
7 |  | 
Which is not true of naturally occurring elements? |
|  | A) | There are 91 of them. |
|  | B) | They were formed by the process of nuclear fusion. |
|  | C) | They were formed by the process of nuclear fission. |
|  | D) | Some of them are gases. |
 |
 |
8 |  | 
During the formation of chemical bonds |
|  | A) | the nuclei change. |
|  | B) | atoms are regrouped and transfer protons. |
|  | C) | electrons retain their original positions. |
|  | D) | the nuclei retain their identity. |
 |
 |
9 |  | 
Perform the following calculation, maintaining the correct number of significant figures. Apply scientific notations if necessary: 2.8 x 3.7960 = |
|  | A) | 11 |
|  | B) | 10.62 |
|  | C) | 10.6 |
|  | D) | 10.63 |
 |
 |
10 |  | 
Perform the following calculation, maintaining the correct number of significant figures. Apply scientific notations if necessary: 3.54 x 1276 = |
|  | A) | 4.52 x 103 |
|  | B) | 4517.04 |
|  | C) | 4.517 x 103 |
|  | D) | 4.5 x 103 |
 |
 |
11 |  | 
Perform the following calculation, maintaining the correct number of significant figures. Apply scientific notations if necessary: 18567 / 6.9 = |
|  | A) | 2690.8696 |
|  | B) | 2.7 x 103 |
|  | C) | 2.69 x 103 |
|  | D) | 2.691 x 103 |
 |
 |
12 |  | 
Perform the following calculation, maintaining the correct number of significant figures. Apply scientific notations if necessary: 2.68 + 3.002 = |
|  | A) | 5.7 |
|  | B) | 5.6820 |
|  | C) | 5.682 |
|  | D) | 5.68 |
 |
 |
13 |  | 
Water at 293° F is a |
|  | A) | solid. |
|  | B) | liquid. |
|  | C) | gas. |
|  | D) | plasma. |
 |
 |
14 |  | 
Water at 287° K is a |
|  | A) | solid. |
|  | B) | liquid. |
|  | C) | gas. |
|  | D) | plasma. |
 |
 |
15 |  | 
Identify this element:
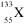 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/39x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/39x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | Cs |
|  | B) | Ba |
|  | C) | Xe |
|  | D) | As |
|  | E) | B |
 |
 |
16 |  | 
Identify this element:
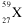 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/40x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/40x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | Co |
|  | B) | Pr |
|  | C) | Hf |
|  | D) | Am |
|  | E) | Cu |
 |
 |
17 |  | 
Identify this element:
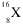 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/41x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/41x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | Ar |
|  | B) | S |
|  | C) | Pm |
|  | D) | O |
|  | E) | Rn |
 |
 |
18 |  | 
Identify this element:
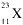 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/42x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072315903/45229/42x.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | Na |
|  | B) | Ti |
|  | C) | V |
|  | D) | Mg |
|  | E) | Al |
 |
 |
19 |  | 
How many protons and neutrons are in this element: 65Zn? |
|  | A) | 30 protons, 35 neutrons |
|  | B) | 30 protons, 65 neutrons |
|  | C) | 35 protons, 30 neutrons |
|  | D) | 65 protons, 35 neutrons |
|  | E) | 65 protons, 30 neutrons |
 |
 |
20 |  | 
How many protons and neutrons are in this element: 195Pt? |
|  | A) | 78 protons, 117 neutrons |
|  | B) | 117 protons, 78 neutrons |
|  | C) | 78 protons, 195 neutrons |
|  | D) | 195 protons, 78 neutrons |
|  | E) | 195 protons, 117 neutrons |
 |
 |
21 |  | 
How many protons, neutrons, and electrons are in 56Fe3+? |
|  | A) | 26 protons, 30 neutrons, 23 electrons |
|  | B) | 26 protons, 30 neutrons, 26 electrons |
|  | C) | 26 protons, 30 neutrons, 29 electrons |
|  | D) | 23 protons, 30 neutrons, 23 electrons |
|  | E) | 26 protons, 56 neutrons, 23 electrons |
 |
 |
22 |  | 
How many protons, neutrons, and electrons are in 28Si3-? |
|  | A) | 17 protons, 14 neutrons, 17 electrons |
|  | B) | 14 protons, 14 neutrons, 14 electrons |
|  | C) | 14 protons, 14 neutrons, 11 electrons |
|  | D) | 14 protons, 14 neutrons, 17 electrons |
|  | E) | 14 protons, 28 neutrons, 17 electrons |
 |

