Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
What is the correct name of the compound shown below?
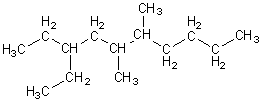 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/Image34.gif','popWin', 'width=312,height=172,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/Image34.gif','popWin', 'width=312,height=172,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | A) | 3-ethyl-4,5-dimethylnonane
|
|  | B) | 5,6-dimethyl-8-ethyldecane
|
|  | C) | 3-ethyl-5,6-dimethyldecane
|
|  | D) | 1,1-diethyl-3,4-dimethyloctane
|
|  | E) | 1-isopropyl-2,4-dimethylheptane
|
 |
 |
2 |  | 
Which of the following are constitutional isomers?
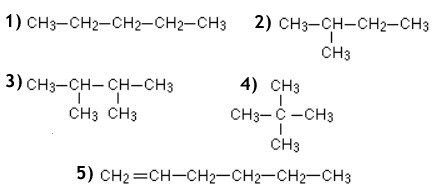 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/Q1521.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (8.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/Q1521.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (8.0K)</a> |
|
|  | A) | 1 and 2
|
|  | B) | 1 and 3
|
|  | C) | 2, 3 and 5
|
|  | D) | 1,2 and 4
|
|  | E) | 1, 3 and 5
|
 |
 |
3 |  | 
Which of the following is a constitutional isomer of CH3CH2COOH that exhibits optical isomerism?
|
|  | A) | CH3CH(OH)COCH3 |
|  | B) | CH3COOCH3 |
|  | C) | CH3COCH2OH
|
|  | D) | HOCH=CHOCH3 |
|  | E) | CH3CH(OH)CHO
|
 |
 |
4 |  | 
What is the IUPAC name of the following compound?
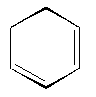 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/image75.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/image75.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | 1,3-cycloheptadiene
|
|  | B) | 3,5-cyclohexadiene
|
|  | C) | 1,3-dicyclohexene
|
|  | D) | 1,3-cyclohexadiene
|
|  | E) | 3,5-cyclopentadiene
|
 |
 |
5 |  | 
Which of the following is likely to be an aromatic compound?
|
|  | A) | C6H5Cl
|
|  | B) | C6H12 |
|  | C) | C2H4Cl2 |
|  | D) | C2H2 |
|  | E) | C4H8 |
 |
 |
6 |  | 
Which of the formulas in the answers represents the alcohol parent of the ester below?
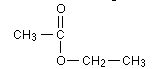 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/chang44.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38224/chang44.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | A) | CH3 - CH2 - CH2 - OH |
|  | B) | CH3 - CH2 - OH |
|  | C) | CH3 - COOH |
|  | D) | CH3 - CH2 - COOH |
|  | E) | none of the above |
 |
 |
7 |  | 
Which type of organic compound contains a hydroxyl group?
|
|  | A) | ethers
|
|  | B) | carboxylic acids
|
|  | C) | esters
|
|  | D) | ketones
|
|  | E) | aldehydes
|
 |
 |
8 |  | 
Which type of organic compound does not contain a carbonyl group?
|
|  | A) | carboxylic acids
|
|  | B) | ethers
|
|  | C) | ketones
|
|  | D) | aldehydes
|
|  | E) | esters
|
 |
 |
9 |  | 
Organic compounds with the general formula HCOOR (where R is a hydrocarbon group) are called:
|
|  | A) | ketones
|
|  | B) | acids
|
|  | C) | ethers
|
|  | D) | esters
|
|  | E) | aldehydes
|
 |
 |
10 |  | 
The segment -CH2CH=CHCH2CH2CH=CHCH2- represents the polymer named
|
|  | A) | polypropylene
|
|  | B) | polybutadiene
|
|  | C) | polystyrene
|
|  | D) | polybutylene
|
|  | E) | polyisoprene
|
 |
 |
11 |  | 
What class of polymers are formed along with small molecules (e.g., water) when monomer units link?
|
|  | A) | condensation
|
|  | B) | addition
|
|  | C) | elimination
|
|  | D) | substitution
|
|  | E) | copolymers
|
 |
 |
12 |  | 
The C-C and C=C bond energies are 346 and 602 kJ/mol. The enthalpy of polymerization of an alkene should be about:
|
|  | A) | 90 kJ/mol
|
|  | B) | -90 kJ/mol
|
|  | C) | -692 kJ/mol
|
|  | D) | -256 kJ/mol
|
|  | E) | none of the above.
|
 |
 |
13 |  | 
What interactions are primarily responsible for stabilizing the helical structure of certain proteins?
|
|  | A) | hydrogen-bonding
|
|  | B) | dispersion forces
|
|  | C) | covalent bonds
|
|  | D) | ionic interactions
|
|  | E) | steric interactions
|
 |
 |
14 |  | 
What two atoms are linked in a peptide bond?
|
|  | A) | C-O
|
|  | B) | N-O
|
|  | C) | S-C
|
|  | D) | C-N
|
|  | E) | C-C
|
 |
 |
15 |  | 
Which of the following arrangements is correct for the interrelation of the protein amino acid sequence, the RNA base sequence, and the DNA base sequence?
|
|  | A) | The DNA base sequence determines the RNA base sequence which, in turn, determines the protein amino acid sequence.
|
|  | B) | The RNA base sequence determines the protein amino acid sequence which, in turn, determines the DNA base sequence.
|
|  | C) | The DNA base sequence determines the protein amino acid sequence which, in turn determines the RNA base sequence.
|
|  | D) | The protein amino acid sequence determines the RNA base sequence which, in turn, determines the DNA base sequence.
|
|  | E) | The RNA base sequence determines the DNA base sequence which, in turn, determines the protein amino acid sequence.
|
 |

