Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
For which of the following reactions is ΔS expected to be positive? |
|  | A) | NH4Cl(s) →
NH3(g) + HCl(g) |
|  | B) | 2H(g) + O(g) →
H2O(g) |
|  | C) | HCl(g) + NaOH(s) →
NaCl(g) + H2O(g) |
|  | D) | 2H2(g) + O2(g) →
2H2O(g) |
|  | E) | N2(g) + 3H2(g) →
2NH3(g) |
 |
 |
2 |  | 
Which of the following changes, all involving one mole of pure water, has the greatest ΔSsys? |
|  | A) | raising the temperature of the solid from –10.0º
C to 0.0º
C |
|  | B) | melting the solid at 0º
C |
|  | C) | raising the temperature of the liquid from 0.0º
C to 10.0º
C |
|  | D) | vaporizing the liquid at 100.0º
C |
|  | E) | raising the temperature of the vapor 100.0º
C to 110.0º
C |
 |
 |
3 |  | 
Calculate ΔSº for the reaction
C9H20(l) + 14O2(g) →
9CO2(g) + 10H2O(g)
Data: Standard molar entropies, Sº in J/mol.K: C9H20(l), 393.7; O2(g), 205.0; CO2(g), 213.7; H2O(g), 188.7 |
|  | A) | -196.3 J/K |
|  | B) | +196.3 J/K |
|  | C) | -546.6 J/K |
|  | D) | +546.6 J/K |
|  | E) | +1341 J/K |
 |
 |
4 |  | 
Select the most likely standard entropy change, ΔS° for the reaction:
2H2(g) + O2(g) →
2H2O(g) |
|  | A) | 0 J/K |
|  | B) | 189 J/K |
|  | C) | -87 J/K |
|  | D) | +44 J/K |
|  | E) | –5 J/K |
 |
 |
5 |  | 
Which of the following statements about entropy and enthalpy of a system is correct? |
|  | A) | The absolute entropy of pure oxygen at 25º
C and 1 atm is zero. |
|  | B) | When ice melts, ΔS is positive and ΔH is negative. |
|  | C) | When a candle burns, ΔS is positive and ΔH is negative. |
|  | D) | The entropy of a system must increase for the reaction to be spontaneous. |
|  | E) | None of the above statements is correct. |
 |
 |
6 |  | 
Which of the following is necessary and sufficient for a process be spontaneous? |
|  | A) | ΔG < 0 |
|  | B) | ΔSsurr > 0 |
|  | C) | ΔH < 0 |
|  | D) | ΔSsys > 0 |
|  | E) | ΔH > TΔS
|
 |
 |
7 |  | 
Given the following reactions and their values of ΔG°:
2CO(g) + O2(g) →
2CO2(g) ΔG° = -516 kJ
4MnO(s) + O2(g) →
2Mn2O3(s) ΔG° = -312 kJ
Calculate ΔG° for the reaction:
Mn2O3(s) + CO(g) →
2MnO(s) + CO2(g) |
|  | A) | +204 kJ |
|  | B) | -204 kJ |
|  | C) | +102 kJ |
|  | D) | -102 kJ |
|  | E) | -414 kJ |
 |
 |
8 |  | 
Methanol is used as a high performance fuel. Calculate ΔG° for the combustion reaction:
2CH3OH(l) + 3O2(g) →
2CO2(g) + 4H2O(g)
Data: Standard free energies of formation, ΔG°f (kJ/mol): CH3OH(l), -163 kJ; O2(g), 0; CO2(g), -394; H2O(g), -229 |
|  | A) | +1146 kJ |
|  | B) | +1460 kJ |
|  | C) | -460 kJ |
|  | D) | -945 kJ |
|  | E) | -1378 kJ |
 |
 |
9 |  | 
Consider the reaction:
C9H20(l) + 14O2(g) →
9CO2(g) + 10H2O(g)
For this reaction, ΔSº is +546.6 J/K and ΔHº is -5685 kJ. Use these values to calculate ΔGº. |
|  | A) | +5848 kJ/mol |
|  | B) | -5848 kJ/mol |
|  | C) | +5139 kJ/mol |
|  | D) | -5139 kJ/mol |
|  | E) | +4922 kJ/mol |
 |
 |
10 |  | 
In 1774 Joseph Priestley prepared the element oxygen by heating mercury(II) oxide:
HgO(s) →
Hg(l) + ½O2(g)
For this reaction, ΔHº
= 90.84 kJ and ΔSº
= 108 J/K. Which of the following statements is true? |
|  | A) | The reaction is only spontaneous at low temperatures. |
|  | B) | The reaction is spontaneous at all temperatures. |
|  | C) | ΔGº
becomes less favorable as the temperature is raised. |
|  | D) | The reaction is spontaneous only at high temperatures. |
|  | E) | The reaction is spontaneous under standard conditions at 25°C. |
 |
 |
11 |  | 
Which of the following is true for an exothermic process? |
|  | A) | qsys > 0, ΔSsurr < 0 |
|  | B) | qsys < 0, ΔSsurr < 0 |
|  | C) | qsys < 0, ΔSsurr > 0 |
|  | D) | qsys > 0, ΔSsurr > 0 |
|  | E) | Need to know ΔSsys before deciding which relationship is true. |
 |
 |
12 |  | 
The compound 1-pentanol has an enthalpy of vaporization of 55.5 kJ/mol and an entropy of vaporization of 148 J/K.mol. Calculate its approximate boiling point. |
|  | A) | 102°C |
|  | B) | 375°C |
|  | C) | 45°C |
|  | D) | 25°C |
|  | E) | 93°C |
 |
 |
13 |  | 
The following reaction occurs at 298 K:
SO2(g) + NO2(g) →
SO3(g) + NO(g)
Data: Standard free energies of formation, ΔGºf (kJ/mol): SO2(g), -300.4; SO3(g), -370.4;
NO(g), 86.7; NO2(g), 51.8
For the above reaction, which of the following conclusions is valid? |
|  | A) | K > 1, ΔHº
> 0 |
|  | B) | K > 1, ΔHº
< 0 |
|  | C) | K < 1, ΔHº > 0 |
|  | D) | K < 1, ΔHº < 0 |
|  | E) | none of the above |
 |
 |
14 |  | 
Consider the reaction:
N2(g) + 3H2(g) → 2NH3(g)
The standard free energy change for this reaction, ΔGº,
is -32.9 kJ. Calculate the equilibrium constant, K, at 25º
C. |
|  | A) | 13.3 |
|  | B) | 5.8 x
105 |
|  | C) | 2.5 |
|  | D) | 4.0 x
10-6 |
|  | E) | 9.1 x
108
|
 |
 |
15 |  | 
Calculate the equilibrium constant K for the following reaction at 25°C:
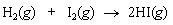 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38229/Q20215a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38229/Q20215a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
Data:
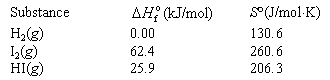 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38229/Q20215b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38229/Q20215b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | A) | 6.85 |
|  | B) | 946 |
|  | C) | 1.06 x
10-3 |
|  | D) | –6.85 |
|  | E) | none of the above |
 |

