Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
A system expands in volume from 2.0 L to 24.5 L at constant temperature. Calculate the work (w) if the expansion occurs against a constant pressure of 5.00 atm. |
|  | A) | -113 J |
|  | B) | 1.24 x 104 J |
|  | C) | -1.14 x 104 J |
|  | D) | 113 J |
|  | E) | 1.14 x 104 J |
 |
 |
2 |  | 
If 1700 cal of heat is added to a system while the system does work equivalent to 3000 cal by expanding against the surrounding atmosphere, the value of ΔE for the process is
|
|  | A) | -1300 cal
|
|  | B) | 4700 cal
|
|  | C) | 1300 cal
|
|  | D) | -4700 cal
|
|  | E) | 2350 cal
|
 |
 |
3 |  | 
Which of the following is not a state function?
|
|  | A) | ΔE |
|  | B) | ΔH |
|  | C) | q |
|  | D) | P |
|  | E) | V |
 |
 |
4 |  | 
In which one of the following processes is ΔH = ΔE?
|
|  | A) | 2HI(g) → H2(g) + I2(g) at atmospheric pressure.
|
|  | B) | Two moles of ammonia gas are cooled from 325 °C to 300 °C at 1.2 atm.
|
|  | C) | H2O(l) → H2O(g) at 100 °C at atmospheric pressure.
|
|  | D) | CaCO3(s) → CaO(s) + CO2(g) at 800 °C at atmospheric pressure.
|
|  | E) | CO2(s) → CO2(g) at atmospheric pressure.
|
 |
 |
5 |  | 
Data:
(1) H2(g) + ½O2(g) → H2O(g) ΔH = -241.8 kJ
(2) H2(g) + ½O2(g) → H2O(l) ΔH = -285.8 kJ
On the basis of the above data, which of the following statements is false?
|
|  | A) | Reaction (1) is exothermic.
|
|  | B) | Reaction (2) is the formation reaction for H2O(l).
|
|  | C) | The reverse of reaction (2) is endothermic.
|
|  | D) | The energy content of H2O(g) is lower than H2O(l).
|
|  | E) | ΔH for the reaction: H2O(l) → H2O(g) is + 44 kJ/mol.
|
 |
 |
6 |  | 
On a gram for gram basis, which of the following substances will release the most energy when it is burned?
|
|  | A) | 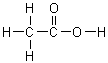 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | B) | 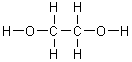 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | C) | 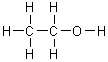 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | D) | 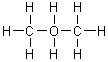 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|  | E) | 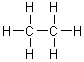 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/Image5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
 |
 |
7 |  | 
What is the amount of heat necessary to raise the temperature of 8.5 kg of water from 12.5 °C to
84 °C?
|
|  | A) | 3.0 x 103 kJ
|
|  | B) | 36 J
|
|  | C) | 2.5 x 103 kJ
|
|  | D) | 2.5 x 106 kJ
|
|  | E) | 25 kJ
|
 |
 |
8 |  | 
When 50. mL (50. g) of 1.00 M HCl at 22.00 °C is added to 50. mL (50. g) of 1.00 M NaOH at
22.00 °C in a coffee cup calorimeter, the temperature increases to 28.87 °C. What is the heat (q) of the reaction between HCl and NaOH? (The specific heat of the solution produced is 4.18 J/g·°C.)
|
|  | A) | -6.3 x 103 J
|
|  | B) | -3.2 x 103 J
|
|  | C) | -2.9 x 103 J
|
|  | D) | -1.7 x 103 J
|
|  | E) | -1.4 x 103 J
|
 |
 |
9 |  | 
A hydrocarbon sample was burned in a bomb calorimeter. The temperature of the calorimeter and the 1.00 kg of water rose from 20.45 °C to 23.65 °C. The heat capacity of the calorimeter, excluding the water, is 2.21 kJ/°C. Using this information, determine the heat released by the combustion.
|
|  | A) | 13.3 kJ
|
|  | B) | 20.5 kJ
|
|  | C) | 46.0 kJ
|
|  | D) | 86.8 kJ
|
|  | E) | none of the above
|
 |
 |
10 |  | 
Data: SO2(g) + ½O2(g) → SO3(g) ΔH° = -99kJ
Use the data above to calculate the standard enthalpy change for the reaction below.
Reaction: 2SO3(g) → O2(g) + 2SO2(g) ΔH° = ?
|
|  | A) | 99 kJ
|
|  | B) | -198 kJ
|
|  | C) | 49.5 kJ
|
|  | D) | -99 kJ
|
|  | E) | 198 kJ
|
 |
 |
11 |  | 
Data: SO2(g) + ½O2(g) → SO3(g) ΔH = -99.1 kJ
Given the above data, calculate the enthalpy change ΔH when 89.6 g of SO2 is converted to SO3.
|
|  | A) | -69.3 kJ
|
|  | B) | -139 kJ
|
|  | C) | 69.3 kJ
|
|  | D) | 139 kJ
|
|  | E) | -111 kJ
|
 |
 |
12 |  | 
Data:
C(graphite) + O2(g) → CO2(g) ΔH° = -393.5 kJ
H2(g) + ½O2(g) → H2O(l) ΔH° = -285.8 kJ
CH3OH(l) + 3/2O2(g) → CO2(g) + 2H2O(l) ΔH° = -726.4 kJ
Using the data above, calculate the enthalpy change for the reaction below.
Reaction: C(graphite) + 2H2(g) + ½O2(g) → CH3OH(l)
|
|  | A) | +238.7 kJ
|
|  | B) | -238.7 kJ
|
|  | C) | +548.3 kJ
|
|  | D) | -548.3 kJ
|
|  | E) | +904.5 kJ
|
 |
 |
13 |  | 
Data:
2Ba(s) + O2(g) → 2BaO(s) ΔH° = -1107.0 kJ
Ba(s) + CO2(g) + ½O2(g) → BaCO3(s) ΔH° = -822.5 kJ
Given the data above, calculate ΔH° for the reaction below.
Reaction: BaCO3(s) → BaO(s) + CO2(g)
|
|  | A) | -1929.5 kJ
|
|  | B) | -1376.0 kJ
|
|  | C) | -284.5 kJ
|
|  | D) | 269.0 kJ
|
|  | E) | 537 kJ
|
 |
 |
14 |  | 
Which one of the following reactions occurring at 25 °C is the formation reaction of H2SO4(l)?
|
|  | A) | H2(g) + S(s) + 2O2(g) → H2SO4(l)
|
|  | B) | H2SO4(l) → H2(g) + S(s) + 2O2(g)
|
|  | C) | H2(g) + S(g) + 2O2(g) → H2SO4(l)
|
|  | D) | H2SO4(l) → 2H(g) + S(s) + 4O(g)
|
|  | E) | 2H(g) + S(g) + 4O(g) → H2SO4(l)
|
 |
 |
15 |  | 
Data: ΔH°f values: CH4(g), -74.8 kJ; CO2(g), -393.5 kJ; H2O(l), -285.8 kJ.
Using the ΔH°f data above, calculate ΔH°rxn for the reaction below.
Reaction: CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
|
|  | A) | -604.2 kJ
|
|  | B) | 890.3 kJ
|
|  | C) | -997.7 kJ
|
|  | D) | -890.3 kJ
|
|  | E) | none of the above
|
 |

