Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
Most of the light emitted by excited mercury atoms has wavelengths of 185, 254, 365, 436, 546 and 615 nm. Which one of the following frequencies is emitted by mercury atoms? |
|  | A) | 1.4 x 1014s-1 |
|  | B) | 4.1 x 1014s-1 |
|  | C) | 8.2 x 1014s-1 |
|  | D) | 8.2 x 1015s-1 |
|  | E) | 4.1 x 1015s-1 |
 |
 |
2 |  | 
How many photons of light having a wavelength of 656 nm are necessary to provide 1.0 J of energy? |
|  | A) | 3.5 x 10-28 |
|  | B) | 3.0 x 10-19 |
|  | C) | 2.8 x 1027 |
|  | D) | 3.3 x 1018 |
|  | E) | none of the above |
 |
 |
3 |  | 
As the temperature of a "black body" increases, |
|  | A) | the average energy of emitted photons increases. |
|  | B) | the average wavelength of emitted photons increases. |
|  | C) | the average frequency of emitted photons decreases. |
|  | D) | the total intensity of emitted light decreases. |
|  | E) | there is no change in the distribution of photons of various energies. |
 |
 |
4 |  | 
Which of the following statements about the absorbance of a colored compound in solution in a spectrometer cell is correct? |
|  | A) | The absorbance will double if the concentration of the colored compound is doubled. |
|  | B) | The absorbance will double if the concentration of the colored compound is halved. |
|  | C) | The absorbance does not depend on the concentration of the colored compound. |
|  | D) | The absorbance is highest for light of the same color as that of the solution. |
|  | E) | The wavelength of the peak in the absorption spectrum will increase if the concentration of the colored compound is increased. |
 |
 |
5 |  | 
For the series of spectral lines of atomic hydrogen with n1 = 3, which of the following wavelengths does not correspond to a wavelength predicted by the Rydberg equation?
Rydberg equation: 1/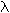 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/lamda.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> = (1.097 x 107 m-1) x (1/n12 - 1/n22) <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/lamda.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> = (1.097 x 107 m-1) x (1/n12 - 1/n22) |
|  | A) | 1875 nm |
|  | B) | 1282 nm |
|  | C) | 1184 nm |
|  | D) | 1094 nm |
|  | E) | 1005 nm |
 |
 |
6 |  | 
Which of the following statements about Bohr's model of the hydrogen atom is correct? |
|  | A) | The radius of an orbit is proportional to the quantum number n. |
|  | B) | A photon is emitted when the electron moves from the ground state to an excited state. |
|  | C) | An orbit is a three dimensional region of space in which the electron is likely to be found. |
|  | D) | The smaller the orbit, the higher the energy of the electron. |
|  | E) | None of the above statements is correct. |
 |
 |
7 |  | 
The energy of an electron in the first Bohr orbit (ground state) of the hydrogen atom is
-13.6 eV. Which one of the following is a possible excited state for the electron?
Recall the equation En = -constant x (1/n2) |
|  | A) | -3.4 eV |
|  | B) | -6.8 eV |
|  | C) | -1.7 eV |
|  | D) | +13.6 eV |
|  | E) | -54.4 eV |
 |
 |
8 |  | 
According to the Bohr theory, what is the ionization energy of atomic hydrogen in kJ/mol?
Bohr equation: E = (-2.18 x 10-18 J)(1/n2) |
|  | A) | 3.62 x 10-42 kJ/mol |
|  | B) | 3.62 x 10-45 J/mol |
|  | C) | 1.31 x 109 J/mol |
|  | D) | 1.31 x 103 kJ/mol |
|  | E) | none of the above |
 |
 |
9 |  | 
What is the de Broglie wavelength of an electron moving at the speed of light? |
|  | A) | 2.2 x 10-42 m |
|  | B) | 2.4 x 10-15 m |
|  | C) | 2.4 x 10-12 m |
|  | D) | 7.3 x 10-4 m |
|  | E) | 4.1 x 1011 m |
 |
 |
10 |  | 
According to the Heisenberg uncertainty principle, if the uncertainty in the speed of a proton is
3.5 x 103 m/s, the uncertainty in its position (in m) is at least |
|  | A) | 1.7 x 10-8 m |
|  | B) | 9.0 x 10-12 m |
|  | C) | 1.5 x 10-38 m |
|  | D) | 6.6 x 1037 m |
|  | E) | none of the above |
 |
 |
11 |  | 
For a hydrogen atom in its ground state, the wave function 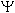 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | A) | equals zero at the nucleus. |
|  | B) | approaches zero at very large values of r. |
|  | C) | has its largest values only in the xy plane. |
|  | D) | has its largest values only in the xz plane. |
|  | E) | goes through a minimum at intermediate values of r. |
 |
 |
12 |  | 
According to the quantum mechanical treatment of the hydrogen atom, which set of quantum numbers is not allowed? |
|  | A) | n = 3, l = 2, ml = 0 |
|  | B) | n = 3, l = 0, ml = 0 |
|  | C) | n = 3, l = 1, ml = 1 |
|  | D) | n = 3, l = 1, ml = -1 |
|  | E) | n = 3, l = 1, ml = 2 |
 |
 |
13 |  | 
For an excited hydrogen atom with the quantum number n equal to 9, which of the following statements is true? |
|  | A) | The energy of the atom is less than the energy for the state in which n is equal to 8. |
|  | B) | If l = 0, there are nine possible values for the magnetic quantum number ml. |
|  | C) | The smallest value of the magnetic quantum number ml is -9. |
|  | D) | The electron must be in one of the p orbitals. |
|  | E) | The angular momentum quantum number l can have any of the values 0, 1, 2, 3, 4, 5, 6, 7, 8. |
 |
 |
14 |  | 
For a pz orbital, the wave function 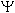 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> is negative in one lobe and positive in the other lobe. Which of the following statements about this orbital is correct? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> is negative in one lobe and positive in the other lobe. Which of the following statements about this orbital is correct? |
|  | A) | The xy plane divides a three-dimensional plot of 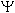 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> into two identical mirror-image halves. <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> into two identical mirror-image halves. |
|  | B) | The xy plane divides a three-dimensional plot of 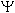 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 into two identical mirror-image halves. <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 into two identical mirror-image halves. |
|  | C) | The xz plane is a nodal surface. |
|  | D) | The yz plane is a nodal surface. |
|  | E) | The radial probability distribution is negative in one lobe and positive in the other. |
 |
 |
15 |  | 
For a 3dx2-y2 orbital, |
|  | A) | the value of 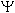 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 is zero everywhere on the y axis. <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 is zero everywhere on the y axis. |
|  | B) | the value of 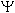 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 is zero everywhere on the z axis. <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/wave.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> 2 is zero everywhere on the z axis. |
|  | C) | the xy plane is a nodal plane. |
|  | D) | the xz plane is a nodal plane. |
|  | E) | the yz plane is a nodal plane. |
 |

