 |
| 1 |  | 
Air (cp = 1.005 kJ/kg-k) is heated from 27°C to 327°C. How much does the specific internal energy of the air change as a result of this heating? |
|  | A) | 301.5 kJ/kg decrease |
|  | B) | 301.5 kJ/kg increase |
|  | C) | 215.4 kJ/kg decrease |
|  | D) | 215.4 kJ/kg increase |
|
|
 |
| 2 |  | 
Air is expanded from 1 MPa, 327°C to 200 kPa in a closed piston-cylinder device executing a PV1.2 = constant process. The work produced during this process is: |
|  | A) | 202.6 kJ/kg |
|  | B) | 263.4 kJ/kg |
|  | C) | 361.7 kJ/kg |
|  | D) | 422.8 kJ/kg |
|
|
 |
| 3 |  | 
Oxygen (M = 32 kg/kg-mol) at 200 kPa, 27°C is contained in a piston-cylinder device arranged to maintain a constant pressure. How much work is produced by this system when it is heated to 227°C? |
|  | A) | 0 kJ/kg |
|  | B) | 11.2 kJ/kg |
|  | C) | 37.1 kJ/kg |
|  | D) | 52.0 kJ/kg |
|
|
 |
| 4 |  | 
A closed system undergoes the series of quasi-equilibrium processes shown here.
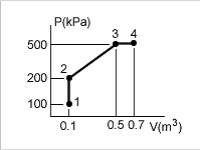 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240298/formula12.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (18.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240298/formula12.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (18.0K)</a> |
|  | A) | 40 kJ |
|  | B) | 140 kJ |
|  | C) | 240 kJ |
|  | D) | 340 kJ |
|
|
 |
| 5 |  | 
Air at 1 MPa, 27°C is contained in a piston-cylinder device that is arranged to maintain a constant pressure. How much heat is required to raise the temperature of this air to 527°C? |
|  | A) | 180 KJ/kg |
|  | B) | 370 KJ/kg |
|  | C) | 520 KJ/kg |
|  | D) | 1040 KJ/kg |
|
|
 |
| 6 |  | 
Two kilograms of steam at 2 MPa, 250°C are contained in a rigid vessel. How much heat must be removed from this vessel to cool it to 25°C? |
|  | A) | Q12,in = 5030 kJ |
|  | B) | Q12,in = 2512 kJ |
|  | C) | Q12,in = -2512 kJ |
|  | D) | Q12,in = -5030 kJ |
|
|
 |
| 7 |  | 
Air is compressed in an insulated piston-cylinder device. Using constant specific heats and treating the process as internally reversible, the amount of work required to compress this air from 100 kPa, 27°C to 2 MPa, 706°C is: |
|  | A) | q12,in = 418 kJ/kg |
|  | B) | q12,in = 512 kJ/kg |
|  | C) | q12,in = 721 kJ/kg |
|  | D) | q12,in = 1030 kJ/kg |
|
|
 |
| 8 |  | 
A piston-cylinder device contains 1 kg of R-134a at 0.8 MPa and 70ºC. Heat is transferred to the refrigerant at constant pressure until the temperature rises to 100ºC. The amount of heat transferred is |
|  | A) | 27.3 kJ |
|  | B) | 30.4 kJ |
|  | C) | 24.0 kJ |
|  | D) | 21.6 kJ |
|
|
 |
| 9 |  | 
Water is heated on an electrical range with a power rating of 1.5 kW for a period of 18 min. The initial and final temperatures of the water are 15ºC and 85ºC and 70 percent of electrical heat is transferred to the water. What is the amount of water? |
|  | A) | 1.4 kg |
|  | B) | 3.9 kg |
|  | C) | 5.5 kg |
|  | D) | 9.2 kg |
|
|
 |
| 10 |  | 
A piston-cylinder device contains 1 kg hydrogen gas. Heat is transferred to the hydrogen until its temperature increases by 10ºC. What is the boundary work done during this process? |
|  | A) | 143 kJ |
|  | B) | 102 kJ |
|  | C) | 80 kJ |
|  | D) | 41 kJ |
|
|

