 |
1 |  | 
An element is a solid, shiny, malleable, and ductile at room temperature. What other properties would it most likely have? |
|  | A) | It conducts both heat and electricity well. |
|  | B) | It conducts heat well, but not electricity. |
|  | C) | It conducts electricity well, but not heat. |
|  | D) | It does not conduct heat or electricity. |
 |
 |
2 |  | 
What is the best description of electrons in the element shown below?
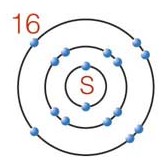 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q23.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (8.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q23.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (8.0K)</a>
|
|  | A) | The electrons fill three energy levels. |
|  | B) | There are three valence electrons. |
|  | C) | There is one stable octet. |
|  | D) | There are two valence electrons. |
 |
 |
3 |  | 
What feature is common to elements in the same group (vertical column) on the periodic table? |
|  | A) | The atoms have the same number of protons. |
|  | B) | The atoms have the same number of energy levels. |
|  | C) | The atoms have the number of valence electrons. |
|  | D) | The atoms have the number of neutrons. |
 |
 |
4 |  | 
Predict the number of valence electrons and their behaviour in an atom of rubidium (atomic number 37). Rubidium is just below potassium on the periodic table.
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q25.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (22.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q25.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (22.0K)</a>
|
|  | A) | The atom has 1 valence electron and gains 7 electrons in chemical reactions. |
|  | B) | The atom has 1 valence electron and loses it in chemical reactions. |
|  | C) | The atom has 7 valence electrons and gains 1 more electron in chemical reactions. |
|  | D) | The atom has 7 valence electrons and loses all of them in chemical reactions. |
 |
 |
5 |  | 
What does an atom that loses electrons become? |
|  | A) | a positively charged ion called a cation |
|  | B) | a negatively charged ion called a cation |
|  | C) | a positively charged ion called an anion |
|  | D) | a negatively charged ion called an anion |
 |
 |
6 |  | 
What are the proper names for the ions shown in the diagram?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q27.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q27.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | aluminium ion and oxygen ion |
|  | B) | aluminide ion and oxygen ion |
|  | C) | aluminide ion and oxide ion |
|  | D) | aluminium ion and oxide ion |
 |
 |
7 |  | 
What happens when an atom of potassium reacts with an atom of fluorine to form the ionic compound potassium fluoride, KF? |
|  | A) | An electron is transferred from the metallic atom to the non-metallic atom. |
|  | B) | An electron is transferred from the non-metallic atom to the metallic atom? |
|  | C) | A single electron is shared. |
|  | D) | Several electrons are shared. |
 |
 |
8 |  | 
What type of binary compound would the chemical CF4 most likely be? |
|  | A) | ionic, because it is formed from a metal and a non-metal |
|  | B) | ionic, because it is formed from two non-metals |
|  | C) | molecular, because it is formed from a metal and a non-metal |
|  | D) | molecular, because it is formed from two non-metals |
 |
 |
9 |  | 
Which description is most appropriate for a sample of the compound sulfur dioxide, SO2? |
|  | A) | a crystal lattice formed by electron transfer |
|  | B) | a crystal lattice formed by electron sharing |
|  | C) | independent molecules formed by electron transfer |
|  | D) | independent molecules formed by electron sharing |
 |
 |
10 |  | 
What is the maximum number of covalent bonds a phosphorus atom can form?
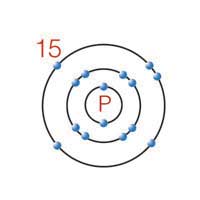 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q31_200.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0070890862/141725/SF10_CH01_Q31_200.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a>
|
|  | A) | 1 |
|  | B) | 3 |
|  | C) | 7 |
|  | D) | 15 |
 |

