 |
1 |  | 
Which isotope has 6 neutrons? |
|  | A) | 136C |
|  | B) | 63Li |
|  | C) | 158O |
|  | D) | 137N |
 |
 |
2 |  | 
Which is true concerning CFCs or Freons? |
|  | A) | Freon 11 and 12 react photochemically with ultraviolet light to produce chlorine atoms in the atmosphere. |
|  | B) | Freons were used because they are non-toxic, non-flammable, and virtually inert. |
|  | C) | The use of recycled Feon-11 and 12 is allowed in the U.S., but tightly regulated. |
|  | D) | All of the above statements are true. |
 |
 |
3 |  | 
Ozone is important because it absorbs some wavelengths of light that enter the atmosphere. Which is true concerning ozone and the biological effects of UV light? |
|  | A) | Ozone has the same structure as carbon dioxide, with two double bonds (O=O=O). |
|  | B) | Ozone absorbs light below 300 nm, the spectral region in which biological damage is maximum. |
|  | C) | Ozone is harmful biologically and is considered a pollutant in the upper atmosphere. |
|  | D) | A and B are true. |
 |
 |
4 |  | 
Which is true concerning ozone concentrations in the atmosphere? |
|  | A) | The ozone concentration has decreased substantially (i.e. 20%) since the early 1940's. |
|  | B) | The ozone concentration is rather constant over the course of a year, changing by only 1 or 2 ppm. |
|  | C) | The ozone concentration is largely in equilibrium with carbon monoxide, due to the carbon cycle. |
|  | D) | All of these statements are true. |
 |
 |
5 |  | 
A diatomic molecule has the structure shown. Based on the use of outer shell electrons, what is a reasonable identity for "X" in the structure?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | A) | X = Ne |
|  | B) | X = F |
|  | C) | X = O |
|  | D) | X = N |
 |
 |
6 |  | 
For these three parts of the electromagnetic spectrum, which is the correct order of increasing wavelength? |
|  | A) | visible, ultraviolet, infrared |
|  | B) | infrared, visible, ultraviolet |
|  | C) | ultraviolet, visible, infrared |
|  | D) | ultraviolet, infrared, visible |
 |
 |
7 |  | 
Which region of the atmosphere contains ozone that helps to protect us from ultraviolet rays, the "good" ozone? |
|  | A) | ionosphere |
|  | B) | mesosphere |
|  | C) | stratosphere |
|  | D) | troposphere |
 |
 |
8 |  | 
How many protons, neutrons, and electrons are in a neutral atom of 6429Cu? |
|  | A) | protons = 64, neutrons = 29, electrons = 29 |
|  | B) | protons = 35, neutrons = 29, electrons = 35 |
|  | C) | protons = 29, neutrons = 35, electrons = 29 |
|  | D) | protons = 29, neutrons = 64, electrons = 35 |
 |
 |
9 |  | 
What is the correct number of outer electrons and the Lewis (electron-dot) structure for carbon monoxide, CO? |
|  | A) | 10 e- and
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) | 14 e- and
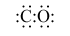 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 10 e- and
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) | 14 e- and
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A9_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
10 |  | 
Which statement comparing these two waves in the electomagnetic spectrum is correct?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | Wave 1 has a lower frequency and travels at the same forward speed as Wave 2. |
|  | B) | Wave 1 has a higher frequency and travels at the same forward speed as Wave 2. |
|  | C) | Wave 1 has a lower frequency and travels at a faster forward speed than Wave 2. |
|  | D) | Wave 1 has a higher frequency and travels at a slower forward speed than Wave 2. |
 |
 |
11 |  | 
Which of these forms of solar radiation has the lowest potential for damage to biological systems? |
|  | A) | infrared radiation |
|  | B) | microwaves |
|  | C) | visible radiation |
|  | D) | X-rays |
 |
 |
12 |  | 
What was the Montreal Protocol's main strategy for reversing stratospheric ozone depletion? |
|  | A) | eliminating illegal CFCs from being imported or exported |
|  | B) | requiring developing and developed countries to follow the same restrictions on CFCs |
|  | C) | improving computer modeling of the upper atmosphere to monitor CFCs |
|  | D) | limiting the production and use of CFCS |
 |
 |
13 |  | 
Which subatomic particles are found in the nucleus of an atom? |
|  | A) | protons and neutrons |
|  | B) | protons and electrons |
|  | C) | neutrons and electrons |
|  | D) | protons, neutrons, and electrons |
 |
 |
14 |  | 
Which is the correct Lewis symbol for an atom of carbon? |
|  | A) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A14_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
15 |  | 
For any region of the electromagnetic spectrum, the energy of a photon is |
|  | A) | directly proportional to the frequency. |
|  | B) | directly proportional to the wavelength. |
|  | C) | directly proportional to the speed of light. |
|  | D) | directly proportional to Planck's constant, h. |
 |
 |
16 |  | 
What is represented by these sphere equations showing the three steps of the Chapman cycle? Each sphere represents an oxygen atom.
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A16.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51616/2A16.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a>
|
|  | A) | They represent the destruction of stratospheric ozone caused by the chemical attack from CFC free radicals. This is a disturbance of the normal process. |
|  | B) | They represent the release of Cl free radicals after they have reacted with oxygen, disturbing the steady state of ozone formation in the stratosphere. |
|  | C) | They represent the dynamic equilibrium between the formation of stratospheric ozone and its destruction. This is the normal steady-state process. |
|  | D) | They represent the normal process that produces excess ozone in the stratosphere when no CFCs or other ozone-depleting substances are present. |
 |

