 |
1 |  | 
"Natural" sources of radiation that represent human exposure exclude? |
|  | A) | solar flames |
|  | B) | cosmic rays |
|  | C) | diagnostic x-rays |
|  | D) | radon |
 |
 |
2 |  | 
Which statement is correct concerning nuclear fusion? |
|  | A) | Nuclear fusion provides energy for the sun and most U.S. nuclear power plants. |
|  | B) | Nuclear fusion produces massive amounts of energy but requires special containment and a temperature of millions of degrees Celsius to initiate. |
|  | C) | Nuclear fusion is the basis of the PEM fuel cell. |
|  | D) | Statements A and B are correct. |
 |
 |
3 |  | 
Given that there are no electrons in the nucleus, how is it possible for an electron to be emitted from the nucleus of a naturally radioactive isotope? |
|  | A) | A gamma ray is thought to break into a proton, which stays in the nucleus to increase the atomic number by one, and a neutron, which is emitted. |
|  | B) | A proton is thought to break into a neutron, which stays in the nucleus to decrease the atomic number by one, and an electron, which is emitted. |
|  | C) | A beta particle is thought to break into a proton, which stays in the nucleus to increase the atomic number by one, and a neutron, which is emitted. |
|  | D) | A neutron is thought to break into a proton, which stays in the nucleus to increase the atomic number by one, and an electron, which is emitted. |
 |
 |
4 |  | 
Sr-90 is a radioactive fission product that contaminated milk in parts of eastern Europe for some time after the accident at Chernobyl. This radioisotope is one of the products formed if a neutron bombards a U-235 nucleus. Three other neutrons are produced as well as another element. What is that element? |
|  | A) | antimony, Sb |
|  | B) | plutonium, Pu |
|  | C) | thorium, Th |
|  | D) | xenon, Xe |
 |
 |
5 |  | 
Co-60 (half-life 5.27 years) and Tc-99m (half-life 6.02 hours) are two isotopes commonly used in nuclear medicine. Both are gamma emitters. Which statement best describes their functions? |
|  | A) | Co-60 is used mainly for therapeutic purposes, which Tc-99m is most often used for diagnostic purposes. |
|  | B) | Tc-99m is used mainly for therapeutic purposes, while Co-60 is most often used for diagnostic purposes. |
|  | C) | Both Co-60 and Tc-99m are used for imaging such things as bone marrow and cardiac functions. |
|  | D) | Both Co-60 and Tc-99m are used in the "gamma knife" developed in Sweden in 1968. |
 |
 |
6 |  | 
On average, the largest contributor to background radiation in the U.S. from man-made sources is |
|  | A) | consumer products, such as smoke detectors. |
|  | B) | occupational exposure while mining uranium. |
|  | C) | X-rays used for medicinal purposes. |
|  | D) | radiation escaping from nuclear power plants. |
 |
 |
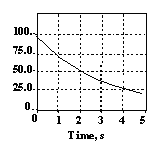
7 |  | 
What is the half-life of the radioisotope X shown in this graph?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | 4 s |
|  | B) | 3 s |
|  | C) | 2 s |
|  | D) | 1 s |
 |
 |
8 |  | 
9038Sr undergoes radioactive decay by beta emission. What is the atomic number of the resulting "daughter" nucleus? |
|  | A) | 36 |
|  | B) | 38 |
|  | C) | 39 |
|  | D) | 90 |
 |
 |
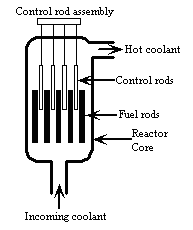
9 |  | 
This diagram shows the reactor core in a typical nuclear power plant. What else is inside a typical containment building in addition to the reactor core?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B9.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B9.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
|
|  | A) | turbines for feeding steam to the generator |
|  | B) | condenser cooling system |
|  | C) | borated water storage tank |
|  | D) | steam generators |
 |
 |
10 |  | 
How many protons and how many neutrons are represented by this symbol?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B11.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51621/7B11.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 92 protons and 235 neutrons |
|  | B) | 92 protons and 143 neutrons |
|  | C) | 143 protons and 92 neutrons |
|  | D) | 235 protons and 92 neutrons |
 |
 |
11 |  | 
U-235 and U-238 are examples of |
|  | A) | allotropes of U |
|  | B) | isotopes of U |
|  | C) | isomers of U |
|  | D) | different elements |
 |
 |
12 |  | 
Which reaction represents a fission reaction? |
|  | A) | 21H + 31H ®42He + 10n + g |
|  | B) | 23892U ®23490Th + 42He + g |
|  | C) | 23994Pu + 210n®24195Am + 0-1e + g |
|  | D) | 23592U + 10n®9037Rb + 14455Cs + 210n + g |
 |
 |
13 |  | 
What is meant by the term "critical" fission reaction? |
|  | A) | Excess neutrons are produced and the result is likely to be a nuclear explosion. |
|  | B) | There is an immediate need to add more nuclear fuel to sustain the fission reaction. |
|  | C) | Borated water must be added to the reactor core to prevent potential meltdown. |
|  | D) | Enough fission occurs so that the chain reaction can be sustained. |
 |
 |
14 |  | 
Nuclear accidents with significant radiation leaks and many human fatalities have occurred at which locations? |
|  | A) | Three Mile Island |
|  | B) | Chernobyl |
|  | C) | Seabrook |
|  | D) | Nuclear accidents involving significant radiation leaks and many human fatalities have occurred at all three facilities. |
 |
 |
15 |  | 
The fuel used in a nuclear power station is |
|  | A) | 23994Pu in PuF6 |
|  | B) | 21H and 31H in H2O |
|  | C) | 23592U in UO2 |
|  | D) | 105B in H2O |
 |
 |
16 |  | 
In the relationship E = mc2, the letter "m" represents |
|  | A) | the mass of the products in a nuclear reaction. |
|  | B) | the mass of the reactants in a nuclear reaction. |
|  | C) | the difference between the sums of the masses of reactants and products in a nuclear reaction. |
|  | D) | the total of the masses of the reactants and products in a nuclear reaction. |
 |

