 |
| 1 |  | 
The empirical formula of a compound |
|  | A) | is also known as the simplest formula |
|  | B) | shows the lowest whole number ration of the elements in a compound |
|  | C) | can be the same as the molecular formula |
|  | D) | all of the above are true |
|
|
 |
| 2 |  | 
Which of the following is an empirical formula? |
|  | A) | C3H6O2 |
|  | B) | C2H6 |
|  | C) | C2H2.5 |
|  | D) | C2H6O2 |
|
|
 |
| 3 |  | 
Which statement is not true? |
|  | A) | It is possible for different compounds to have the same empirical formula. |
|  | B) | Compounds with the same empirical formulas have the same properties. |
|  | C) | The empirical formula of an ionic compound is usually the same as its chemical formula. |
|  | D) | An empirical formula may be the same as the molecular formula. |
|
|
 |
| 4 |  | 
What is the relationship between the subscripts of a molecular formula and those in an empirical formula (n is whole number)? |
|  | A) | molecular formula subscripts = n x emiprical formula subscripts |
|  | B) | empirical formula subscripts = n x molecular formula subscripts |
|  | C) | 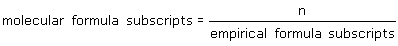 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q4c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q4c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | D) | 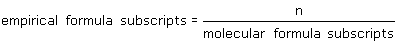 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q4d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q4d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|
 |
| 5 |  | 
You are calculating an empirical formula and arrive at an answer of C1H2.67. What do you do next? |
|  | A) | Nothing. The answer is acceptable. |
|  | B) | Start over. You have obviously made a mistake. |
|  | C) | Convert 2.67 to  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q5cd.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q5cd.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | D) | Convert 2.67 to  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q5cd.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> and multiply both subscripts by 3 to get the lowest whole number subscript. <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ2_Q5cd.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> and multiply both subscripts by 3 to get the lowest whole number subscript.
|
|
|
 |
| 6 |  | 
In order to calculate an empirical formula you need to know |
|  | A) | the percent composition data |
|  | B) | the molar mass of the elements |
|  | C) | the molar mass of the compound |
|  | D) | percentage composition data and the molar mass of the elements |
|
|
 |
| 7 |  | 
When calculating the empirical formula of a compound that is 32.3% Ca, 33.3% P and 34.4% O, for simplicity's sake, in the first step assume that you have |
|  | A) | 1 mol of the compound |
|  | B) | 50 g of the compound |
|  | C) | 100 g of the compound |
|  | D) | 100 mol of the compound |
|
|
 |
| 8 |  | 
In the above question, you determine that the elements are present in the molar ratio of Ca - 0.8059, P - 1.075 and O - 2.150. What is the empirical formula? |
|  | A) | Use the calculated ratios as subscripts. The empirical formula is C0.8059P11.075O2.150. |
|  | B) | Round the ratios to the nearest whole number and use as subscripts. The empirical formula is CPO2. |
|  | C) | Divide the molar amounts by the smallest one and use the quotients as the subscripts. The empirical formula is CP1.32O2.67. |
|  | D) | Divide the molar amounts by the smallest one. Multiply each by 3 to obtain whole numbers. The empirical formula is C3P4O8. |
|
|

