 |
| 1 |  | 
The molecular formula of a compound |
|  | A) | is also known as the simplest formula. |
|  | B) | shows the lowest whole number ratio of the elements in the compound. |
|  | C) | shows the actual number of each type of element in the compound. |
|  | D) | is always different from the empirical formula. |
|
|
 |
| 2 |  | 
In order to find a molecular formula you need to know |
|  | A) | either the percentage composition and the molar mass or the empirical formula and the molar mass. |
|  | B) | the percentage composition of the compound. |
|  | C) | the empirical formula of the compound. |
|  | D) | the molar mass of the compound. |
|
|
 |
| 3 |  | 
A compound whose molar mass is 88.12 g/mol has the empirical formula C2H4O. What is its molecular formula? |
|  | A) | C2H4O |
|  | B) | C4H8O2 |
|  | C) | C6H12O3 |
|  | D) | There is not enough information to answer the question. |
|
|
 |
| 4 |  | 
The carbon-hydrogen combustion analyzer is an instrument used to determine |
|  | A) | the molar mass of a compound. |
|  | B) | the percent composition of a compound. |
|  | C) | the empirical formula of a compound. |
|  | D) | the number of C and H atoms in a compound. |
|
|
 |
| 5 |  | 
An ionic compound that incorporates water molecules into its crystal structure is called |
|  | A) | a hydride |
|  | B) | aqueous. |
|  | C) | anhydrous. |
|  | D) | a hydrate. |
|
|
 |
| 6 |  | 
What is the molar mass of BaCl2.2H2O? |
|  | A) | 208.23 g/mol |
|  | B) | 226.25 g/mol |
|  | C) | 228.27 g/mol |
|  | D) | 244.27 g/mol |
|
|
 |
| 7 |  | 
What is percent water in BaCl2.2H2O? |
|  | A) | 7.375% |
|  | B) | 7.965% |
|  | C) | 14.75% |
|  | D) | 15.93% |
|
|
 |
| 8 |  | 
An experiment to determine the chemical formula of a hydrate Z reveals that for every 0.178 mol of Z there are 0.534 mol of water. What is the formula of the hydrate? |
|  | A) | 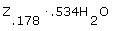 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | B) | 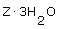 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | D) | 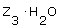 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128781/atlCHEMISTRY_CH03_QUIZ3_Q8d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|

