 |
| 1 |  | 
In a double displacement reaction |
|  | A) | two ionic compounds react by exchanging ions |
|  | B) | a precipitate may be formed |
|  | C) | a gas may be produced |
|  | D) | all of the above |
|
|
 |
| 2 |  | 
Which combination of solutions will result in the formation of a precipitate? |
|  | A) | Ba(NO3)2(aq) + Na2CO3(aq) |
|  | B) | Ca(NO3)2(aq) + NaCl(aq) |
|  | C) | KNO3(aq) + Na2CO3(aq) |
|  | D) | NH4NO3(aq) + NaCl(aq) |
|
|
 |
| 3 |  | 
A metal carbonate reacts with an acid to produce a gas. Which gas is formed? |
|  | A) | N2(g) |
|  | B) | CO2(g) |
|  | C) | CO(g) |
|  | D) | H2O(g) |
|
|
 |
| 4 |  | 
Which of the following is one of the products of a reaction between an acid and a base? |
|  | A) | Water |
|  | B) | Hydronium ion |
|  | C) | Hydroxide ion |
|  | D) | Hydrogen gas |
|
|
 |
| 5 |  | 
Identify the spectator ions in the following total ionic equation:
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | A) | Na+ and OH- |
|  | B) | OH- and Ag+ |
|  | C) | Ag+ and (NO3)- |
|  | D) | Na+ and (NO3)- |
|
|
 |
| 6 |  | 
The following equation is an example of what type of equation?
2Ag+(aq) + S2-(aq) → Ag2S(s) |
|  | A) | Molecular equation |
|  | B) | Total ionic equation |
|  | C) | Net ionic equation |
|  | D) | Skeleton equation |
|
|
 |
| 7 |  | 
In writing the total ionic equation for the reaction between sodium phosphate and copper(II) chloride, which compound is not written as it's dissociated ions? |
|  | A) | Sodium chloride |
|  | B) | Copper(II) phosphate |
|  | C) | Copper(II) chloride |
|  | D) | Sodium phosphate |
|
|
 |
| 8 |  | 
The net ionic equation for the reaction that occurs from mixing a solution of barium chloride with a solution of potassium sulfate is: |
|  | A) | 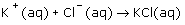 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | D) | 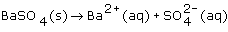 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ2_Q8d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|
 |
| 9 |  | 
What is qualitative analysis? |
|  | A) | It determines how much of a compound, element or ion is in an unknown sample. |
|  | B) | It identifies compounds, elements or ions in an unknown solution. |
|  | C) | It measures concentrations of ions. |
|  | D) | None of the above. |
|
|

