 |
| 1 |  | 
What is the dissociation equation for an aqueous solution of magnesium chloride? |
|  | A) | 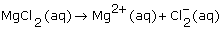 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1a.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | B) | 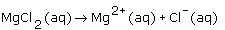 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | C) | 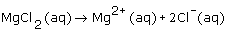 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1c.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | D) | 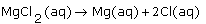 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0070938539/128786/atlCHEMISTRY_CH08_QUIZ3_Q1d.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|
|
 |
| 2 |  | 
What is the concentration of nitrate ions in a 0.15 mol/L solution of Fe(NO3)3? |
|  | A) | 0.45 mol/L |
|  | B) | 0.30 mol/L |
|  | C) | 0.15 mol/L |
|  | D) | There is not enough information to answer the question. |
|
|
 |
| 3 |  | 
What is the concentration of a solution of aluminum sulfate (Al2(SO4)3) if the concentration of sulfate ions is 0.60 mol/L/? |
|  | A) | 1.2 mol/L |
|  | B) | 0.60 mol/L |
|  | C) | 0.40 mol/L |
|  | D) | 0.20 mol/L |
|
|
 |
| 4 |  | 
Equal volumes of 0.20 mol/L NaCl and 0.30 mol/L KCl are mixed together. What is the concentration of chloride ions in the mixture? |
|  | A) | 0.20 mol/L |
|  | B) | 0.25 mol/L |
|  | C) | 0.30 mol/L |
|  | D) | You need to know the volumes of the original solutions to answer the question. |
|
|
 |
| 5 |  | 
What volume of a 0.500 mol/L solution of Na2CO3 contains enough carbonate ions to completely precipitate 0.0150 mol of Ba2+ions? |
|  | A) | 300 mL |
|  | B) | 90.0 mL |
|  | C) | 30.0 mL |
|  | D) | 10.0 mL |
|
|
 |
| 6 |  | 
What volume of a 1.50 mol/L solution of Ba(OH)2 contains enough hydroxide ions to completely precipitate the iron(II) ions found in 60.0 mL of a 0.500 mol/L solution of FeCl2? |
|  | A) | 180. mL |
|  | B) | 60.0 mL |
|  | C) | 20.0 mL |
|  | D) | 12.5 mL |
|
|
 |
| 7 |  | 
How many moles of precipitate BaSO4 are formed when 50.0 mL of a 0.500 mol/L solution of BaCl2 is mixed with 35.0 mL of a 0.500 mol/L solution of Na2SO4? |
|  | A) | 0.0175 mol |
|  | B) | 0.0250 mol |
|  | C) | 0.0350 mol |
|  | D) | 0.0500 mol |
|
|
 |
| 8 |  | 
How many moles of Mg(OH)2 will be precipitated when 75.0 mL of 0.400 mol/L MgCl2 is mixed with 75.0 mL of 0.400 mol/L NaOH? |
|  | A) | 0.0750 mol |
|  | B) | 0.0600 mol |
|  | C) | 0.0300 mol |
|  | D) | 0.0150 mol |
|
|
 |
| 9 |  | 
In the above question, what mass of precipitate is formed? |
|  | A) | 0.875 g |
|  | B) | 1.75 g |
|  | C) | 3.50 g |
|  | D) | 4.37 g |
|
|

