Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
1 |  | 
The lone pair of electrons on sulfur in the sulfite ion (SO32-) is in
|
|  | A) | a p orbital.
|
|  | B) | an s orbital.
|
|  | C) | an sp2 hybrid orbital.
|
|  | D) | an sp3 hybrid orbital.
|
|  | E) | none of the above orbital types.
|
 |
 |
2 |  | 
Which has sp3d hybridization and no dipole moment?
|
|  | A) | PCl3 |
|  | B) | BF3 |
|  | C) | SF4 |
|  | D) | BrF3 |
|  | E) | PCl5 |
 |
 |
3 |  | 
Which of the following hybridization patterns is associated with octahedral geometry?
|
|  | A) | sp2 |
|  | B) | sp3 |
|  | C) | sp3d2 |
|  | D) | sp3d |
|  | E) | sp |
 |
 |
4 |  | 
Which hybrid orbitals are associated with bond angles of 90° and 120° at the central atom?
|
|  | A) | sp3 |
|  | B) | sp3d2 |
|  | C) | sp |
|  | D) | sp2 |
|  | E) | sp3d |
 |
 |
5 |  | 
Which carbon orbitals would be involved in the formation of the hybrid orbitals that are present in ethyne (acetylene), HCCH?
|
|  | A) | 1s, 2s, 2px |
|  | B) | 1s, 2s, 2px, 2py |
|  | C) | 2s, 2px |
|  | D) | 2s, 2px, 2py |
|  | E) | 2s, 2px, 2py, 2pz |
 |
 |
6 |  | 
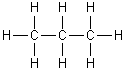
For the following molecule, what is the hybridization for each carbon?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38215/11_2_6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/38215/11_2_6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | A) | sp |
|  | B) | sp5 |
|  | C) | sd4 |
|  | D) | sp2 |
|  | E) | sp3 |
 |
 |
7 |  | 
In H2CNH, which orbitals overlap in forming the NC bond?
|
|  | A) | 1s overlaps with 2s |
|  | B) | sp overlaps with 1s |
|  | C) | sp2 overlaps with 1s |
|  | D) | sp2 overlaps with sp2 |
|  | E) | sp2 overlaps with sp2 and 2p overlaps with 2p |
 |
 |
8 |  | 
The carbon atoms in ethyne (acetylene, C2H2) are joined by a triple bond. The 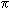 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> bonds in this compound consist of: <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> bonds in this compound consist of:
|
|  | A) | 6 electrons, 3 orbitals, and 5 lobes
|
|  | B) | 6 electrons, 3 orbitals, and 4 lobes
|
|  | C) | 4 electrons, 3 orbitals, and 4 lobes
|
|  | D) | 4 electrons, 2 orbitals, and 2 lobes
|
|  | E) | 4 electrons, 2 orbitals, and 4 lobes
|
 |
 |
9 |  | 
Which of the following molecules will not have a 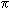 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> bond? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> bond?
|
|  | A) | C2H2 |
|  | B) | C2H6 |
|  | C) | N2H2 |
|  | D) | None of the above.
|
|  | E) | All of the above.
|
 |
 |
10 |  | 
Which of the substituted ethylene molecules listed can exist in both cis- and trans- forms?
|
|  | A) | H2C=CH2 |
|  | B) | H2C=CF2 |
|  | C) | HFC=CF2 |
|  | D) | HFC=CHF
|
|  | E) | HFC=CH2 |
 |
 |
11 |  | 
According to molecular orbital theory, in the O2+ ion, how many valence electrons are in antibonding molecular orbitals?
|
|  | A) | 6
|
|  | B) | 5
|
|  | C) | 4
|
|  | D) | 3
|
|  | E) | 2
|
 |
 |
12 |  | 
Which is the correct order for the oxygen-oxygen bond length?
|
|  | A) | HOOH>O2->O2+>O2 |
|  | B) | HOOH>O2->O2>O2+ |
|  | C) | HOOH<O2+<O2-<O2 |
|  | D) | HOOH<O2-<O2+<O2 |
|  | E) | HOOH>O2->O2+>O2 |
 |
 |
13 |  | 
Assume that N2 absorbs a photon and an electron is raised to the first excited state.
|
|  | A) | The excited N2 is less stable than N2 in the ground state
|
|  | B) | The excited N2 is more stable than N2 in the ground state
|
|  | C) | The bond length of N2 in the excited state is shorter than the bond length of N2 in the ground state.
|
|  | D) | The excited state molecule will break up into two nitrogen atoms.
|
|  | E) | none of the above
|
 |
 |
14 |  | 
The bond order in NO predicted by molecular orbital theory is
|
|  | A) | 1
|
|  | B) | 1.5
|
|  | C) | 2
|
|  | D) | 2.5
|
|  | E) | none of the above.
|
 |
 |
15 |  | 
In the molecular orbital description of bonding in benzene (C6H6), how many electrons occupy
delocalized 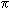 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> MOs? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0072396814/37547/pi.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> MOs?
|
|  | A) | 2
|
|  | B) | 3
|
|  | C) | 4
|
|  | D) | 5
|
|  | E) | 6
|
 |

