 |
| 1 |  | 
Saturated water vapor at 150 kPa is condensed to saturated liquid in a steady-flow, isobaric heat exchanger. The released heat is transferred to the surrounding air whose temperature is 20°C. The increase of the entropy associated with this process is |
|  | A) | -4.731 kJ/kg-K |
|  | B) | -2.366 kJ/kg-K |
|  | C) | 2.366 kJ/kg-K |
|  | D) | 4.731 kJ/kg-K |
|
|
 |
| 2 |  | 
Steam at 2 MPa, 300°C is expanded in a steady-flow, adiabatic turbine to 30 kPa. What is the lowest possible temperature at the outlet of this turbine? |
|  | A) | 69.1°C |
|  | B) | 101.1°C |
|  | C) | 150.7°C |
|  | D) | 203.2°C |
|
|
 |
| 3 |  | 
Steam at 2 MPa, 300°C is expanded through a steady-flow, adiabatic turbine to 30 kPa. How much work does this turbine produce? |
|  | A) | 478.7 kJ/kg |
|  | B) | 523.2 kJ/kg |
|  | C) | 639.2 kJ/kg |
|  | D) | 741.6 kJ/kg |
|
|
 |
| 4 |  | 
Which temperature-specific entropy state diagram below correctly represents the isobaric cooling process occurring in a heat exchanger?
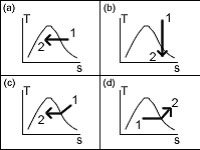 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula16.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (23.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula16.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (23.0K)</a> |
|  | A) | a |
|  | B) | b |
|  | C) | c |
|  | D) | d |
|
|
 |
| 5 |  | 
The total amount of heat added to a closed system executing the reversible, isothermal process shown below is given by:
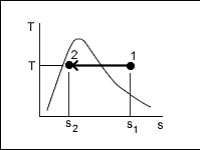 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula17.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula17.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> |
|  | A) | Qin = T (s2 - s1) |
|  | B) | Qin = T (s1 - s2) |
|  | C) | Qin = mT (s2 - s1) |
|  | D) | Qin = mT (s1 - s2) |
|
|
 |
| 6 |  | 
According to the Tds equation, the specific entropy change of air when it is heated from T1 to T2 while executing an isobaric process is: |
|  | A) | cvln (T2 / T1) |
|  | B) | cPln (T2 / T1) |
|  | C) | cvln (T2 / T1) - R ln (v2 / v1) |
|  | D) | cPln (T2 / T1) - R ln (v2 / v1) |
|
|
 |
| 7 |  | 
Air at 5 MPa, 967°C is expanded through a steady-flow device to 100 kPa, 27°C. What is the change in the specific entropy of the air? |
|  | A) | -1.372 kJ/kg-K |
|  | B) | -0.269 kJ/kg-K |
|  | C) | 1.742 kJ/kg-K |
|  | D) | 2.638 kJ/kg-K |
|
|
 |
| 8 |  | 
A 0.5-kg steel (C = 0.5 kJ/kg-k) rivet cools from 800 K to 300 K upon being installed in a riveted building structure. The entropy change of this rivet is: |
|  | A) | -0.631 kJ/K |
|  | B) | -0.245 kJ/K |
|  | C) | 0.245kJ/K |
|  | D) | 0.631 kJ/K |
|
|
 |
| 9 |  | 
Oxygen at 100 kPa, 27°C is compressed to 1 MPa in an adiabatic compressor whose isentropic efficiency is 0.80. The oxygen temperature at the compressor outlet is: |
|  | A) | 376 K |
|  | B) | 421 K |
|  | C) | 566 K |
|  | D) | 649 K |
|
|
 |
| 10 |  | 
Water undergoes the reversible process illustrated here as it passes through a steady-flow device that has one outlet and one outlet. How much work does this device produce?
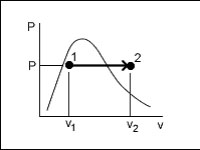 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula18.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240301/formula18.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (16.0K)</a> |
|  | A) | 0 kJ/kg |
|  | B) | P (v2 - v1) kJ/kg |
|  | C) | R (T2 - T1) kJ/kg |
|  | D) | cv (T2 - T1) kJ/kg |
|
|

