Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
| 1 |  | 
Name the following compound:
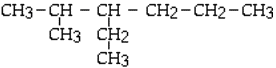 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/15_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/15_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | A) | 4-ethyl-5-methylhexane |
|  | B) | 2-methyl-3-propylpentane |
|  | C) | 3-ethyl-2-methylhexane |
|  | D) | 4-methyl-3-propylpentane |
|  | E) | 2-methyl-3-ethylhexane |
|
|
 |
| 2 |  | 
Which of the following is not a constitutional isomer of CH3CH2COOH? |
|  | A) | CH3CH2COOCH3 |
|  | B) | CH3COOCH3 |
|  | C) | CH3COCH2OH |
|  | D) | HOCH=CHOCH3 |
|  | E) | CH3CH(OH)CHO |
|
|
 |
| 3 |  | 
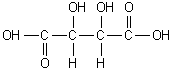 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/Image35.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/Image35.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
Tartaric acid, shown above, is a dihydroxycarboxylic acid. How many chiral centers does it possess? |
|  | A) | 0 |
|  | B) | 1 |
|  | C) | 2 |
|  | D) | 3 |
|  | E) | 4 |
|
|
 |
| 4 |  | 
Which, if any, of the following are geometric isomers?
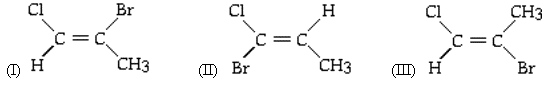 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/15_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38224/15_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (3.0K)</a> |
|  | A) | I and II |
|  | B) | II and III |
|  | C) | I and III |
|  | D) | None of them are geometric isomers. |
|  | E) | All of them are geometric isomers. |
|
|
 |
| 5 |  | 
What is the general formula for alkynes? |
|  | A) | CnH2n+2 |
|  | B) | C2nH2n |
|  | C) | CnH2n-2 |
|  | D) | CnHn-2 |
|  | E) | CnH2n |
|
|
 |
| 6 |  | 
Which, if any, of the following compounds will be the major product(s) of the addition of Br2 to CH3 - CH = CH2? |
|  | A) | CH3Br and CH2 = CHBr |
|  | B) | CH3 - CBr = CHBr and HBr |
|  | C) | CH3 - CHBr - CH2Br and HBr |
|  | D) | CH3 - CBr2 - CH3 and HBr |
|  | E) | CH3 - CHBr - CH2Br |
|
|
 |
| 7 |  | 
What type of compound is represented by the formula CH3CH2CH2CH2CH2CH2OH? |
|  | A) | an alkene |
|  | B) | an alcohol |
|  | C) | an alkyne |
|  | D) | an unsaturated hydrocarbon |
|  | E) | a CFC |
|
|
 |
| 8 |  | 
Which one of the following is the formula for a ketone? |
|  | A) | CH3CH2CHO |
|  | B) | CH3OCH3 |
|  | C) | CH3CH2COCH3 |
|  | D) | CH3CH2COOH |
|  | E) | CH3CH2COOCH3 |
|
|
 |
| 9 |  | 
Which of the following is a constitutional isomer of, and has a much lower boiling point than CH3CH2COOH? |
|  | A) | CH3CH2COOCH3 |
|  | B) | CH3COOCH3 |
|  | C) | CH3COCH2OH |
|  | D) | HOCH=CHOCH3 |
|  | E) | CH3CH(OH)CHO |
|
|
 |
| 10 |  | 
Which of the following is the monomer of the polymer used to coat cooking utensils with non-stick surfaces? |
|  | A) | CH2=CHCl |
|  | B) | CF2=CF2 |
|  | C) | CH2=CF2 |
|  | D) | H2C=CHCN |
|  | E) | H2C=CH-C6H5 |
|
|
 |
| 11 |  | 
Which of the following polymers is a condensation polymer? |
|  | A) | polypropylene |
|  | B) | polyester |
|  | C) | poly(vinyl chloride) |
|  | D) | Teflon® |
|  | E) | polyacrylonitrile |
|
|
 |
| 12 |  | 
What type of functional group links the monomer units in nylon-66? |
|  | A) | amine |
|  | B) | ether |
|  | C) | carbonyl |
|  | D) | alkene |
|  | E) | amide |
|
|
 |
| 13 |  | 
A protein is |
|  | A) | a naturally-occurring addition polymer. |
|  | B) | a polymer of amino acid units. |
|  | C) | a polymer containing the genetic code. |
|  | D) | a polysaccharide. |
|  | E) | a polymer consisting of many nucleotide units. |
|
|
 |
| 14 |  | 
Which, if any, of the following is the general structure of an amino acid? |
|  | A) | H2N-COOH |
|  | B) | H2NCH2COOH |
|  | C) | RCH2CONH2 |
|  | D) | H2NCH2CHRCOOH |
|  | E) | H2NCHRCOOH |
|
|
 |
| 15 |  | 
DNA consists of repeating units, nucleotides, each of which consists of |
|  | A) | a base and a phosphate group. |
|  | B) | a phosphate group, a deoxyribose unit, and an amino acid. |
|  | C) | a phosphate group, a deoxyribose unit, and a base. |
|  | D) | a phosphate group, a sugar unit, and an acid. |
|  | E) | a phosphate group, an amino acid unit, and a sugar. |
|
|

