Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
| 1 |  | 
For the overall hypothetical reaction A + 5B →
4C, the rate of appearance of C given by Δ[C]/Δt is the same as |
|  | A) | Δ[A]/Δt |
|  | B) | -(5/4)(Δ[B]/Δt) |
|  | C) | -(4/5)(Δ[B]/Δt) |
|  | D) | -(1/4)(Δ[A]/Δt) |
|  | E) | none of the above. |
|
|
 |
| 2 |  | 
When heated, the compound RX3 decomposes to a mixture of products. The following data were collected for the decomposition at 100°C. What is the average rate of reaction, -Δ
[RX3]/Δt, over the entire experiment?
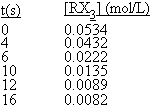 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38225/ch16_1_02.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/38225/ch16_1_02.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | A) | 0.0028 mol/L.s |
|  | B) | 0.045 mol/L.s |
|  | C) | 0.0014 mol/L.s |
|  | D) | -0.0027 mol/L.s |
|  | E) | 0.0057 mol/L.s |
|
|
 |
| 3 |  | 
The initial rate of the reaction
PCl5 →
PCl3 + Cl2
is increased a factor of four when the concentration of PCl5is doubled. Therefore, the rate |
|  | A) | depends on the concentrations of PCl3 and Cl2. |
|  | B) | is first order with respect to PCl5. |
|  | C) | is second order with respect to PCl5. |
|  | D) | is fourth order with respect to PCl5. |
|  | E) | is first order with respect to PCl3. |
|
|
 |
| 4 |  | 
Bromine atoms react with iso-butane (iso-C4H10) to form hydrogen bromide and the t-butyl radical
(t-C4H9): Br + iso-C4H10 → HBr + t-C4H9.
The reaction is first order with respect to each of the reactants, and at 298 K, the rate constant is 1.02 x 106 L mol-1 s-1. Calculate the initial rate of the reaction in a 4.0 L reaction vessel containing 0.60 moles of iso-butane and 0.00010 moles of bromine atoms. |
|  | A) | 1.5 mol L-1 s-1 |
|  | B) | 3.8 mol L-1 s-1 |
|  | C) | 2.5 x 1011 mol L-1 s-1 |
|  | D) | 2.5 x 102 mol L-1 s-1 |
|  | E) | 6.7 x 102 mol L-1 s-1 |
|
|
 |
| 5 |  | 
Consider the thermal decomposition of cyclobutane (C4H8(g)) at 438°C,
C4H8(g) → 2C2H4(g).
The reaction follows first-order kinetics and the rate constant is k = 2.48 x 10-4 s-1. If the initial concentration of cyclobutane is 0.800 mol/L, what concentration will remain after 10.0 min? |
|  | A) | 0.689 mol/L |
|  | B) | 0.455 mol/L |
|  | C) | 0.333 mol/L |
|  | D) | 0.248 mol/L |
|  | E) | 0.0061 mol/L |
|
|
 |
| 6 |  | 
A certain first-order reaction is 46% complete in 68 min at 25°C. What is its rate constant? |
|  | A) | 9.1 x 10-3 min-1 |
|  | B) | 1.1 x 10-2 min-1 |
|  | C) | 31 min-1 |
|  | D) | 51 min-1 |
|  | E) | none of the above |
|
|
 |
| 7 |  | 
Consider the reaction A → products. Which, if any, of the following plots is consistent with a second-order reaction? |
|  | A) | ln[A] plotted against time gives a straight line of positive slope. |
|  | B) | ln[A] plotted against time gives a straight line of negative slope. |
|  | C) | 1/[A] plotted against time gives a straight line of positive slope. |
|  | D) | 1/[A] plotted against time gives a straight line of negative slope. |
|  | E) | None of the above plots is consistent with a second-order reaction. |
|
|
 |
| 8 |  | 
Consider the reaction A → products. Which of the following plots is consistent with a zero-order reaction? |
|  | A) | [A] plotted against time gives a horizontal, straight line. |
|  | B) | ln[A] plotted against time gives a straight line of negative slope. |
|  | C) | 1/[A] plotted against time gives a straight line of positive slope. |
|  | D) | [A] plotted against time gives a straight line of negative slope. |
|  | E) | [A] plotted against time gives a curved line of negative slope, decreasing in magnitude as time increases. |
|
|
 |
| 9 |  | 
What is the value of the rate constant for a first-order reaction for which the half-life is 26.7 min? |
|  | A) | 18.5 min-1 |
|  | B) | 38.5 min-1 |
|  | C) | 9.25 min-1 |
|  | D) | 19.3 min-1 |
|  | E) | 0.0260 min-1 |
|
|
 |
| 10 |  | 
The rate constant of a first-order reaction is 3.68 x 10-2 s-1 at 150°C, and the activation energy is 71 kJ/mol.
What is the value of the rate constant at 170°C? |
|  | A) | 9.2 x 10-2 s-1 |
|  | B) | 3.7 x 10-2 s-1 |
|  | C) | 2.49 s-1 |
|  | D) | 4.0 x 10-2 s-1 |
|  | E) | none of the above |
|
|
 |
| 11 |  | 
In order to obtain the activation energy of a reaction using a graphical method, __________ is plotted against _________, giving a straight line whose slope is equal to __________. |
|  | A) | k; T; -Ea |
|  | B) | k; 1/T; -Ea |
|  | C) | lnk; T; -Ea/R |
|  | D) | k; 1/T; -Ea/R |
|  | E) | lnk; 1/T; -Ea/R |
|
|
 |
| 12 |  | 
Select the appropriate rate law for the elementary process shown below.
2A → B + C |
|  | A) | Rate = k[2A] |
|  | B) | Rate = k[A] |
|  | C) | Rate = k[A]2 |
|  | D) | Rate = k[A]1/2 |
|  | E) | Rate = 2k[A] |
|
|
 |
| 13 |  | 
The reaction
3ClO-(aq) → ClO3-(aq)
+ 2Cl-(aq) has been proposed to occur by the following mechanism.
ClO-(aq) + ClO-( aq)→ ClO2-( aq) + Cl-( aq) (slow)
ClO2-(aq) + ClO-(aq) → ClO3-(aq) + Cl-(aq) (fast
Which rate law is consistent with this mechanism? |
|  | A) | rate = k[ClO-] |
|  | B) | rate = k[ClO-]3 |
|  | C) | rate = k[ClO2-][ClO-] |
|  | D) | rate = k[ClO-]2 |
|  | E) | rate = k[Cl-][ClO-]2 |
|
|
 |
| 14 |  | 
Consider the reaction:
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
A suggested mechanism for this reaction follows:
(1) NO(g) + NO(g) → N2O4(g) (slow)
(2) N2O4(g) + H2(g) → N2(g) + H2O2 (g) (fast)
(3) H2O2(g) + H2(g) → 2H2O(g) (fast)
Based on this mechanism, which, if any, of the following actions will not affect the rate of the reaction? |
|  | A) | adding a catalyst |
|  | B) | adding more NO(g) |
|  | C) | adding more H2(g) |
|  | D) | increasing the temperature |
|  | E) | All of these will affect the rate of the reaction. |
|
|
 |
| 15 |  | 
A catalyst speeds up a reaction by |
|  | A) | increasing the number of high-energy molecules. |
|  | B) | increasing the temperature of the molecules in the reaction. |
|  | C) | increasing the number of collisions between molecules. |
|  | D) | increasing the activation energy for the reaction. |
|  | E) | providing a new reaction pathway for molecules. |
|
|

