Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
| 1 |  | 
Select the incorrect statement about elements, compounds and mixtures. |
|  | A) | Elements consist of atoms of the same kind. |
|  | B) | Compounds contain two or more elements, chemically combined. |
|  | C) | The properties of a compound are different from those of its constituent elements. |
|  | D) | A mixture is an example of what is meant by the term "substance". |
|  | E) | Both elements and compounds may exist as molecules. |
|
|
 |
| 2 |  | 
A compound of sulfur and oxygen is 50.0% sulfur, 50.0% oxygen by weight. Sulfur and oxygen form another compound, in accordance with the law of multiple proportions. Which of the following is a likely composition for the second compound? |
|  | A) | 85.0 mass % sulfur 15.0 mass % oxygen. |
|  | B) | 65.0 mass % sulfur 35.0 mass % oxygen. |
|  | C) | 55.0 mass % sulfur 45.0 mass % oxygen. |
|  | D) | 45.0 mass % sulfur 55.0 mass % oxygen. |
|  | E) | 40.0 mass % sulfur 60.0 mass % oxygen. |
|
|
 |
| 3 |  | 
Rutherford's experiment with alpha particle scattering by gold foil established that |
|  | A) | electrons have a negative charge. |
|  | B) | protons are 1840 times heavier than electrons. |
|  | C) | protons are not evenly distributed throughout an atom. |
|  | D) | atoms are made of protons, neutrons and electrons. |
|  | E) | the nucleus contains protons and neutrons. |
|
|
 |
| 4 |  | 
The number of neutrons in an atom of 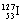 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> is <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> is |
|  | A) | 53 |
|  | B) | 74 |
|  | C) | 126 |
|  | D) | 127 |
|  | E) | 180 |
|
|
 |
| 5 |  | 
The ion 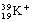 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> has <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> has
| | |
|
|  | A) | 18 electrons, 18 protons, 21 neutrons |
|  | B) | 19 electrons, 18 protons, 20 neutrons |
|  | C) | 18 electrons, 19 protons, 20 neutrons |
|  | D) | 19 electrons, 19 protons, 39 neutrons |
|  | E) | 18 electrons, 19 protons, 19 neutrons |
|
|
 |
| 6 |  | 
Which of the following sets are isoelectronic (i.e., have the same number of electrons)?
i. Br-, Kr, Sr2+
ii. C, N-, O2-
iii. Mg2+, Ca2+, Sr2+
iv. O2-, O, O2+
v. Ag+, Cd2+, Pd |
|  | A) | i and ii |
|  | B) | i and v |
|  | C) | i, iii and iv |
|  | D) | ii, iii and v |
|  | E) | i, iii iv and v |
|
|
 |
| 7 |  | 
The element antimony has an atomic weight of 121.757 amu and only two naturally occurring isotopes. One isotope has an abundance of 57.3% and an isotopic mass of 120.904 amu. What is the mass of the other isotope? |
|  | A) | 52.479 amu |
|  | B) | 121.757 amu |
|  | C) | 122.393 amu |
|  | D) | 122.610 amu |
|  | E) | 122.902 amu |
|
|
 |
| 8 |  | 
For the element bromine, the symbol, group number, group name and physical state are, respectively |
|  | A) | Bo, 17, noble gas, gas. |
|  | B) | B, 13, semimetal, solid. |
|  | C) | Br, 17, halogen, liquid. |
|  | D) | Br, 17, halogen, gas. |
|  | E) | Br, 15, chalcogenide, gas. |
|
|
 |
| 9 |  | 
The hydride ion, H-, occurs in compounds of hydrogen with |
|  | A) | alkali metals. |
|  | B) | halogens. |
|  | C) | chalcogenides. |
|  | D) | non-metals. |
|  | E) | oxygen. |
|
|
 |
| 10 |  | 
Which of the following pairs of elements would be most likely to form an ionic compound? |
|  | A) | P and Br |
|  | B) | Zn and K |
|  | C) | C and O |
|  | D) | Al and Rb |
|  | E) | F and Ca |
|
|
 |
| 11 |  | 
What is the name of NaI? |
|  | A) | sodium iodide |
|  | B) | sodium(I) iodide |
|  | C) | sodium monoiodide |
|  | D) | sodious iodide |
|  | E) | sodium iodine |
|
|
 |
| 12 |  | 
Which of the following combinations of names and formulas is incorrect? |
|  | A) | H3PO4 phosphoric acid |
|  | B) | HNO3 nitric acid |
|  | C) | NaHCO3 sodium carbonate |
|  | D) | H2CO3 carbonic acid |
|  | E) | KOH potassium hydroxide |
|
|
 |
| 13 |  | 
The correct name of the compound N2O4 is |
|  | A) | nitric oxide. |
|  | B) | dinitrogen oxide. |
|  | C) | dinitrogen tetraoxide. |
|  | D) | nitrous oxide. |
|  | E) | nitrogen dioxide. |
|
|
 |
| 14 |  | 
The name for HIO3 is |
|  | A) | hydroiodic acid. |
|  | B) | iodic acid. |
|  | C) | periodic acid. |
|  | D) | hypoiodic acid. |
|  | E) | hydrogen iodide. |
|
|
 |
| 15 |  | 
Which one of the following combinations of names and formulas of ions is incorrect? |
|  | A) | O2- oxide |
|  | B) | Al3+ aluminum |
|  | C) | NO3- nitrate |
|  | D) | PO43- phosphate |
|  | E) | CrO42- chromate |
|
|

