Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
| 1 |  | 
Calculate the wavelength of visible light having a frequency of 4.37 x 1014 s-1. |
|  | A) | 12.0 nm |
|  | B) | 343 nm |
|  | C) | 686 nm |
|  | D) | 674 nm |
|  | E) | none of the above |
|
|
 |
| 2 |  | 
The frequency of electromagnetic radiation of wavelength 5.6 mm is |
|  | A) | 5.4 x 107 Hz |
|  | B) | 1.9 x 10-11 Hz |
|  | C) | 5.4 x 1010 Hz |
|  | D) | 1.1 x 108 Hz |
|  | E) | none of the above |
|
|
 |
| 3 |  | 
The wavelength of a beam of light is 24 mm. What is the energy of one of its photons? |
|  | A) | 5.3 x 10-47 J |
|  | B) | 8.3 x 10-24 J |
|  | C) | 5.3 x 10-45 J |
|  | D) | -8.3 x 10-21 J |
|  | E) | none of the above |
|
|
 |
| 4 |  | 
Refer to the absorption spectrum of chlorophyll a in Figure B7.4 in your textbook. Which of the following statements is false? |
|  | A) | Chlorophyll a absorbs light of wavelengths between 400 and 480 nm. |
|  | B) | Chlorophyll a absorbs light of wavelengths between 640 and 680 nm. |
|  | C) | Chlorophyll a transmits light of wavelengths between 500 and 600 nm. |
|  | D) | Chlorophyll a has a maximum absorbance at a wavelength near 450 nm. |
|  | E) | Chlorophyll a has a maximum transmittance at a wavelength of 663 nm. |
|
|
 |
| 5 |  | 
The Rydberg equation, with n1 = 1, predicts an ultraviolet series of spectral lines of atomic hydrogen. Which of the following wavelengths is not predicted by the equation?
Rydberg equation: 1/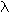 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/lamda.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> = (1.097 x 107 m-1) x (1/n12 - 1/n22) <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/lamda.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> = (1.097 x 107 m-1) x (1/n12 - 1/n22) |
|  | A) | 182 nm |
|  | B) | 103 nm |
|  | C) | 97 nm |
|  | D) | 95 nm |
|  | E) | 91 nm |
|
|
 |
| 6 |  | 
In Bohr's model of the hydrogen atom, the radius of an orbit |
|  | A) | is proportional to n2. |
|  | B) | is smallest for the highest energy state. |
|  | C) | increases when a photon of light is emitted from an excited atom. |
|  | D) | can have any value that is larger than the ground-state radius. |
|  | E) | none of the above |
|
|
 |
| 7 |  | 
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 3 to n = 1 energy level, based on the Bohr theory.
Use the equation En = -2.18 x 10-18 J(1/n2) |
|  | A) | 2.92 x 1015s-1 |
|  | B) | 1.94 x 10-18s-1 |
|  | C) | 3.21 x 1015s-1 |
|  | D) | 3.05 x 10-15s-1 |
|  | E) | Not enough information given to calculate answer. |
|
|
 |
| 8 |  | 
According to Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon?
Use the equation: En = (-2.18 x 10-18 J)(1/n2) |
|  | A) | n = 5 to n = 3 |
|  | B) | n = 6 to n = 1 |
|  | C) | n = 4 to n = 3 |
|  | D) | n = 5 to n = 4 |
|  | E) | n = 6 to n = 5 |
|
|
 |
| 9 |  | 
The de Broglie wavelength for a baseball moving at 25.0 m/s is 2 x 10-34 m. If a baseball could travel at the speed of light, what would be its de Broglie wavelength at that speed? |
|  | A) | 1.7 x 10-41 m |
|  | B) | 1.5 x 10-26 m |
|  | C) | 1.7 x 10-43 m |
|  | D) | 1.5 x 10-28 m |
|  | E) | 1.5 x 10-24 m |
|
|
 |
| 10 |  | 
According to the Heisenberg uncertainty principle, if the uncertainty in the speed of an electron is
3.5 x 103 m/s, the uncertainty in its position (in m) is at least |
|  | A) | 66 m |
|  | B) | 17 m |
|  | C) | 6.6 x 10-8 m |
|  | D) | 1.7 x 10-8 m |
|  | E) | none of the above |
|
|
 |
| 11 |  | 
Which of the following atoms is not a one-electron system? |
|  | A) | H |
|  | B) | He+ |
|  | C) | Li2+ |
|  | D) | Be2+ |
|  | E) | O7+ |
|
|
 |
| 12 |  | 
What value or values of ml are allowable for an orbital with l = 2? |
|  | A) | 0 |
|  | B) | 2 |
|  | C) | -1 |
|  | D) | none of the above |
|  | E) | all of the above |
|
|
 |
| 13 |  | 
According to the quantum-mechanical model, how many orbitals in a given atom have n = 3? |
|  | A) | 4 |
|  | B) | 7 |
|  | C) | 9 |
|  | D) | 10 |
|  | E) | 18 |
|
|
 |
| 14 |  | 
The nodes for a 3s atomic orbital are |
|  | A) | two points near the nucleus and another point at an infinite distance from the nucleus. |
|  | B) | three spherical solids. |
|  | C) | one plane and two spheres. |
|  | D) | two concentric circles. |
|  | E) | two concentric spheres. |
|
|
 |
| 15 |  | 
Consider a 3dxz orbital. Which of the following statements is incorrect? |
|  | A) | The xz plane is a nodal surface. |
|  | B) | The xz plane divides the electron probability distribution into two identical mirror-image halves. |
|  | C) | The xy plane divides the electron probability distribution into two identical mirror-image halves. |
|  | D) | The yz plane divides the electron probability distribution into two identical mirror-image halves. |
|  | E) | The nucleus is located at a node. |
|
|

