 |
| 1 |  | 
Which of the choices below correctly states the descriptions of all three subatomic particles? |
|  | A) | Neutrons are positively charged particles that are found in the nucleus. Electrons are neutral particles that are found in the nucleus. Protons are negatively charged particles that are found in orbitals surrounding the nucleus. |
|  | B) | Electrons are positively charged particles that are found in the nucleus. Protons are neutral particles that are found in the nucleus. Neutrons are negatively charged particles that are found in orbitals surrounding the nucleus. |
|  | C) | Protons are positively charged particles that are found in the nucleus. Neutrons are neutral particles that are found outside the nucleus. Electrons are negatively charged particles that are found in orbitals surrounding the nucleus. |
|  | D) | Protons are positively charged particles that are found in the nucleus. Neutrons are neutral particles that are found in the nucleus. Electrons are negatively charged particles that are found in orbitals surrounding the nucleus. |
|
|
 |
| 2 |  | 
The six atoms that are basic to life are |
|  | A) | carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. |
|  | B) | sodium, potassium, calcium, iron, and magnesium. |
|  | C) | carbon, hydrogen, nitrogen, oxygen, potassium, and sodium. |
|  | D) | chlorine, iron, sodium, calcium, potassium, and carbon. |
|
|
 |
| 3 |  | 
Take a look at the periodic table. Which of these lists is correct regarding the element Magnesium?
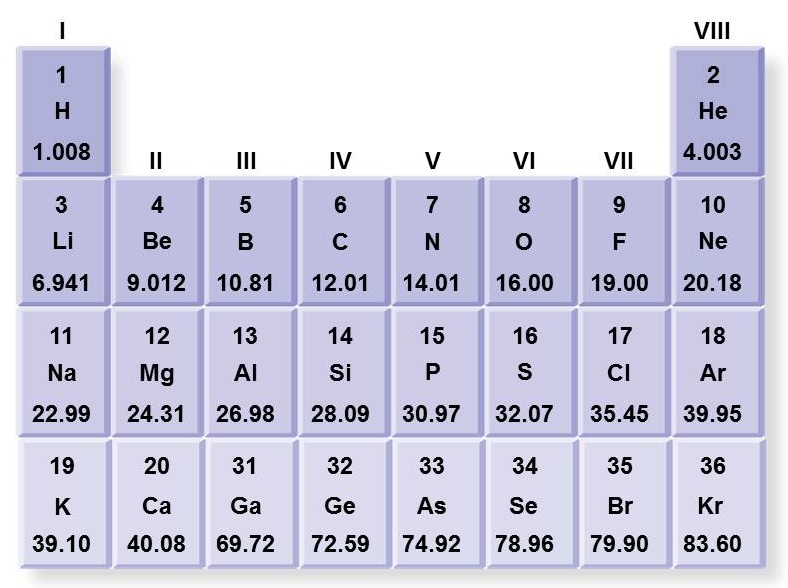 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q3_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (104.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q3_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (104.0K)</a> |
|  | A) | Its atomic number is 24.31, atomic symbol is Mg, and atomic mass is 12. |
|  | B) | Its atomic number is II, atomic symbol is MG, and atomic mass is 24.31. |
|  | C) | Its atomic number is II, atomic symbol is Mg, and atomic mass is 12. |
|  | D) | Its atomic number is 12, atomic symbol is Mg, and atomic mass is 24.31. |
|
|
 |
| 4 |  | 
Which of the following is true concerning an isotope? |
|  | A) | An element and its isotope(s) have different numbers of electrons, the same mass numbers, and the same atomic number. |
|  | B) | An element and its isotope(s) have different numbers of neutrons, different mass numbers, and the same atomic number. |
|  | C) | An element and its isotope(s) have different numbers of neutrons, the same mass number, and the same atomic number. |
|  | D) | An element and its isotope(s) have the same number of neutrons, different mass numbers, and the same atomic number. |
|
|
 |
| 5 |  | 
Which atom is electrically neutral?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> |
|  | A) | A sodium (Na) atom with 11 protons, 12 electrons, and 11 neutrons. |
|  | B) | A sodium (Na) atom with 11 protons, 11 electrons, and 13 neutrons. |
|  | C) | A sodium (Na) atom with 11 protons, 11 electrons, and 12 neutrons. |
|  | D) | A sodium (Na) atom with 11 protons, 10 electrons, and 12 neutrons. |
|
|
 |
| 6 |  | 
How many electrons are in the outer shell of a neutral atom with the atomic number 17?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> |
|  | A) | 2 |
|  | B) | 17 |
|  | C) | 18 |
|  | D) | 7 |
|
|
 |
| 7 |  | 
Most atoms, except for those will full valence electron shells, readily bond with one another. This phenomenon is explained by |
|  | A) | the octet rule. |
|  | B) | the valence electron shell. |
|  | C) | the presence of radioactive isotopes. |
|  | D) | the formation of compounds. |
|
|
 |
| 8 |  | 
Which of the following atoms will form an ionic bond?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> |
|  | A) | two hydrogen (H) atoms |
|  | B) | two oxygen (O) atoms |
|  | C) | one carbon (C) and four hydrogen (H) atoms |
|  | D) | one potassium (K) and one chlorine (Cl) atom |
|
|
 |
| 9 |  | 
Which of the following atoms will form a covalent bond?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073525537/1028583/ch02Post_Q5_Q6_Q8_Q9_ref.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (179.0K)</a> |
|  | A) | one oxygen (O) and two hydrogen (H) atoms |
|  | B) | one sodium (Na) and one chlorine (Cl) atom |
|  | C) | one potassium (K) and one chlorine (Cl) atom |
|  | D) | one lithium (Li) and one bromine (Br) atom |
|
|
 |
| 10 |  | 
A(n) ____________ bond is formed between equally electronegative atoms, which equally share electrons. A(n) ____________ bond is formed between atoms that differ in their electronegativity, which unequally share electrons. |
|  | A) | polar covalent; nonpolar covalent |
|  | B) | hydrogen; ionic |
|  | C) | nonpolar; polar |
|  | D) | ionic; hydrogen |
|
|
 |
| 11 |  | 
Hydrogen bonds are |
|  | A) | individually strong, but collectively weak. |
|  | B) | individually stronger than a single covalent bond. |
|  | C) | individually weak, but collectively strong. |
|  | D) | individually stronger than a double covalent bond. |
|
|
 |
| 12 |  | 
Hydrogen bonds form |
|  | A) | only in water. |
|  | B) | between a negatively charged ion and a positively charged ion. |
|  | C) | within or between molecules in which electropositive hydrogen is attracted to other electropositive atoms. |
|  | D) | within or between molecules in which partially positive hydrogen atoms are attracted to partially negative atoms, like oxygen or nitrogen. |
|
|
 |
| 13 |  | 
Most of the properties of water that make life possible, such as its liquid nature at most temperatures that support life, its expansion upon freezing, its ability to serve as a transport medium, and its ability to take up heat without changing its temperature quickly, can be attributed to |
|  | A) | ionic bonding. |
|  | B) | polar covalent bonding. |
|  | C) | its polarity. |
|  | D) | hydrogen bonding. |
|
|
 |
| 14 |  | 
The bonds between water molecules make water a very unique compound. Molecules of water are __________ (stick to each other) and __________ (stick to other polar materials). This allows water to flow freely, and makes water an excellent transport medium in plants and animals. |
|  | A) | adhesive; cohesive |
|  | B) | hydrophilic; hydrophobic |
|  | C) | hydrophobic; hydrophilic |
|  | D) | cohesive; adhesive |
|
|
 |
| 15 |  | 
Sodium chloride is dissolved in a beaker of water. The water in this situation is called a __________. The sodium chloride is called a __________. |
|  | A) | solution; salt |
|  | B) | dissolver; disolvee |
|  | C) | solvent; solute |
|  | D) | solute; solvent |
|
|
 |
| 16 |  | 
An acidic solution contains an excess of __________ ions. A basic solution contains an excess of __________ ions. |
|  | A) | chloride (Cl−); sodium (Na+) |
|  | B) | hydrogen (H−); hydroxide (OH+) |
|  | C) | hydroxide (OH−); hydrogen(H+) |
|  | D) | hydrogen (H+); hydroxide (OH−) |
|
|
 |
| 17 |  | 
An acid or a base is __________ if it dissociates nearly completely. |
|  | A) | strong |
|  | B) | weak |
|  | C) | a buffer |
|  | D) | a good cleaning agent |
|
|
 |
| 18 |  | 
The pH scale compares the concentration of hydroxide ions to the concentration of hydrogen ions in a solution. Moving down the pH scale from 14 to 0, each unit has 10 times the concentration of __________ than the previous unit. Moving up the scale from 0 to 14, each unit has 10 times the concentration of __________ than the previous unit. |
|  | A) | acid; base |
|  | B) | base; acid |
|  | C) | hydroxide ions; hydrogen ions |
|  | D) | hydrogen ions; hydroxide ions |
|
|
 |
| 19 |  | 
The pH scale ranges from 0 to 14. The difference between a pH value of 1 and 2 is the presence of _________ the concentration of hydroxide ions. A solution of pH 1 is ___________, and a solution of pH 2 is __________. |
|  | A) | 10,000 times; acidic; basic |
|  | B) | 10 times; acidic; acidic |
|  | C) | 100 times; acidic; basic |
|  | D) | 1,000 times; acidic; acidic |
|
|
 |
| 20 |  | 
A buffer is a chemical or combination of chemicals that resists changes in pH and helps keep pH within normal limits. Consider human blood, and how what seem like small changes in pH can have drastic consequences, ranging from cramping to coma. When a buffer such as aspirin is introduced, it helps contribute to which important concept (that you might recall from chapter 1)? |
|  | A) | the acquisition of materials and energy for survival |
|  | B) | natural selection |
|  | C) | homeostasis, or a balanced internal environment |
|  | D) | response to stimuli |
|
|

