 |
1 |  | 
Which is the formula for the hydronium ion? |
|  | A) | OH-(aq) |
|  | B) | H3O+(aq) |
|  | C) | OH2-(aq) |
|  | D) | H+(aq) |
 |
 |
2 |  | 
Which equation represents the reaction of an acid anhydride? |
|  | A) | HNO3(aq) ----> H+(aq) + NO3-(aq) |
|  | B) | Ca(OH)2(aq) + 2 HCl (aq) ----> CaCl2(aq) + 2 H2O (l) |
|  | C) | SO2(g) + H2O (l) ----> H2SO3(aq) |
|  | D) | 2 H+(aq) + CaCO3(s) ----> Ca2+(aq) + CO2(aq) + H2O (l) |
 |
 |
3 |  | 
At any temperature, an aqueous solution is considered basic only if |
|  | A) | [H+] = [OH-] |
|  | B) | [H+] > [OH-] |
|  | C) | [H+] < [OH-] |
|  | D) | [OH-] = 1.0 x 10-7 |
 |
 |
4 |  | 
The term "acid rain" refers to any rain that has a pH below 5.6. "Normal rain" has a pH |
|  | A) | above 7. |
|  | B) | exactly 7. |
|  | C) | below 7. |
|  | D) | between 5.6 and 7. |
 |
 |
5 |  | 
Which of these substances will have the lowest pH? |
|  | A) | cola soft drinks |
|  | B) | lemon juice |
|  | C) | tomato juice |
|  | D) | household ammonia |
 |
 |
6 |  | 
If the concentration of H+(aq) in an aqueous solution is 1 x 10-9 M, what is the pH of the solution? |
|  | A) | 1 |
|  | B) | 5 |
|  | C) | 9 |
|  | D) | 10 |
 |
 |
7 |  | 
What equation represents a neutralization reaction? |
|  | A) | HNO3(aq) ----> H+(aq) + NO3-(aq) |
|  | B) | Ca(OH)2(aq) + 2 HCl (aq) ----> CaCl2(aq) + 2 H2O (l) |
|  | C) | SO2(g) + H2O (l) ----> H2SO3(aq) |
|  | D) | Ca2+(aq) + CO2(g) + H2O (l) ----> 2 H+(aq) + CaCO3(s) |
 |
 |
8 |  | 
CaCO3 is a major chemical component in limestone and marble, materials that can be damaged by acid rain. What is the correct name for CaCO3? |
|  | A) | calcium carbonite |
|  | B) | calcium carboxide |
|  | C) | calcium carbonate |
|  | D) | calcium carbon trioxide |
 |
 |
9 |  | 
What is the major natural source of SOx? |
|  | A) | oceans |
|  | B) | soil and plants |
|  | C) | volcanoes |
|  | D) | lightning |
 |
 |
10 |  | 
What pair of substances reacts with H2O to acidify rain? |
|  | A) | SO3(aq) and O3(g) |
|  | B) | CO (g) and NO (g) |
|  | C) | NH3(g) and CO2(g) |
|  | D) | SO3(g) and NO2(g) |
 |
 |
11 |  | 
Which diagram best represents the composition of a sodium hydroxide solution? (Water molecules are not shown.)
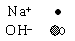 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B11_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
12 |  | 
The 1990 Clean Air Act Amendments set up a system of auctioning SOx emissions. This system for "emissions trading" within an air basin has been |
|  | A) | the major reason that SOx emissions have fallen dramatically in the last ten years. |
|  | B) | an important but minor factor in reducing SOx emissions. The major reason has been switching from high sulfur to low sulfur coal. |
|  | C) | an important but minor factor in reducing SOx emissions. The major reason has been scrubbing coal with limestone. |
|  | D) | an important but minor factor in reducing SOx emissions. The major reason has been pulverizing coal and cleaning it with water. |
 |
 |
13 |  | 
Catalytic converters for automobiles were originally developed to reduce carbon monoxide emissions. Which equation represents the second function of a modern catalytic converter? |
|  | A) | 2 CO (g) + O2(g) ----> 2CO2(g) |
|  | B) | CO2(g) ----> C (g) + O2(g) |
|  | C) | 2 NO (g) ----> N2(g) + O2(g) |
|  | D) | 2 NO (g) + O2(g) ----> 2 NO2(g) |
 |
 |
14 |  | 
Which statement concerning the question of acid rain correctly interprets the nature of the problem? |
|  | A) | Acid rain is a simple problem that can be solved by individual action. |
|  | B) | Acid rain is a somewhat straightforward problem that can be solved by industry alone. |
|  | C) | Acid rain is a somewhat complex problem but can be solved simply by banning all cars. |
|  | D) | Acid rain is a complex problem and will require many different individual and collective actions to solve. |
 |
 |
15 |  | 
A 0.1 M HCl solution is tested for conductivity using this type of apparatus. What do you predict will happen and why?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B15.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51620/6B15.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | The light bulb will not light up. HCl is not soluble in water. |
|  | B) | The light bulb will shine dimly. HCl is only partially ionized in aqueous solution. |
|  | C) | The light bulb will shine brightly. HCl is completely ionized in aqueous solution. |
|  | D) | The light bulb will not light up. HCl is in molecular form in aqueous solution. |
 |
 |
16 |  | 
Which is the "total" ionic equation for the reaction of KOH (aq) with HNO3(aq)? |
|  | A) | K+(aq) + OH-(aq) + H+(aq) + NO3-(aq) ----> H2O (l) + K+(aq) + NO3-(aq) |
|  | B) | KOH (aq) + HNO3(aq) ----> KNO3(aq) + H2O (l) |
|  | C) | KOH (aq) + HNO3(aq) ----> K+(aq) + OH-(aq) + H+(aq) + NO3-(aq) |
|  | D) | OH-(aq) + H+(aq) ----> H2O (l) |
 |
 |
17 |  | 
The major method used to achieve a major drop in SO2 emissions in the U.S. has been |
|  | A) | switching from high sulfur to low sulfur coal. |
|  | B) | scrubbing coal with limestone. |
|  | C) | pulverizing coal and cleaning with water. |
|  | D) | auctioning SO2 allowances. |
 |
 |
18 |  | 
A blood sample has a pH of 7.45. Which of the following describes the proton concentration [H+]? |
|  | A) | between 10-7 and 10-8 M |
|  | B) | between 10-6 and 10-7 M |
|  | C) | between 107 and 108 M |
|  | D) | between 10-4 and 10-5 M |
 |
 |
19 |  | 
The Clean Air Act Amendments of 1990 encouraged coal switching. What is a potential repercussion of the Clean Air Act and coal switching? |
|  | A) | economic (cost of SO2 scrubbers; effects on states that produce high sulfur coal) |
|  | B) | political (appears to benefit some states over others) |
|  | C) | chemical (higher NO2 levels from the low sulfur coal) |
|  | D) | Both A and B are potential repercussions. |
 |
 |
20 |  | 
Acid rain has deleterious effects on many objects. Which contains an incorrectly paired objects and the effect that acid rain has on them? |
|  | A) | marble and granite; solubility of carbonate salts |
|  | B) | steel; increased oxidation |
|  | C) | the health of a human's lungs; allergy to gaseous SO2 |
|  | D) | trees; growth reduction and leaf loss |
 |

