 |
1 |  | 
2 H2(g) + O2(g) ----> 2 H2O (l)
This reaction represents the |
|  | A) | reaction that takes place in the most common type of storage battery. |
|  | B) | reaction that takes place in most alkaline cells, such as the DuracellÓ battery. |
|  | C) | overall reaction that takes place in one type of fuel cell. |
|  | D) | electrolysis of water in an electrolytic cell. |
 |
 |
2 |  | 
As the lithium-iodide cell, Li + 1/2 I2 ----> LiI, discharges, lithium is oxidized and iodine is reduced. Which is the correct half-reaction of oxidation? |
|  | A) | Li + e- ----> Li+ |
|  | B) | Li+ ---->
Li + e- |
|  | C) | Li ---->
Li+ + e- |
|  | D) | Li+ + e- ----> Li |
 |
 |
3 |  | 
Which of these is not a problem with moving towards a "hydrogen economy"? |
|  | A) | finding raw material containing hydrogen |
|  | B) | separating hydrogen from raw material |
|  | C) | storing hydrogen safely |
|  | D) | transporting hydrogen economically |
 |
 |
4 |  | 
Hydrogen gas can be made by reacting steam with coke, producing H2 and CO. Which statement about this process is true? |
|  | A) | The reaction is exothermic and needs improved catalysts to increase the yield. |
|  | B) | The reaction is endothermic and must be carried out at high temperatures. |
|  | C) | The reaction must be carried out using the process of electrolysis, which requires energy. |
|  | D) | The products are quite unstable and often an uncontrolled explosion results. |
 |
 |
5 |  | 
This equation represents an important redox reaction.
PbO2(s) + Pb (s) + 2 H2SO4(aq) ----> 2 PbSO4(s) + 2 H2O (aq)
Where is this reaction used in commercial application? |
|  | A) | alkaline cells used in calculators |
|  | B) | solar cells at remote power stations |
|  | C) | storage battery in most automobiles |
|  | D) | photovoltaic cells for direct conversion of sunlight |
 |
 |
6 |  | 
What type of battery or cell would likely be used to power this portable drill?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (5.0K)</a>
|
|  | A) | a mercury oxide button battery |
|  | B) | a lead storage battery |
|  | C) | a nickel-cadmium battery |
|  | D) | a hydrogen-oxygen fuel cell |
 |
 |
7 |  | 
Why is silicon the principal element used in constructing solar cells? |
|  | A) | Si is abundant. It is found in 25% of the earth's crust. |
|  | B) | Si acts as a semiconductor when hit by a photon of infrared or visible light. |
|  | C) | Si is in the same family with C and has four outer electrons. |
|  | D) | Si exists as separate, non-bonded atoms, making electron transfer relatively easy. |
 |
 |
8 |  | 
What does this Daimler-Chrysler NeCar-4 use to generate power?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (23.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (23.0K)</a>
|
|  | A) | methanol-based fuel cells |
|  | B) | lead-acid storage batteries |
|  | C) | photovoltaic cells |
|  | D) | fission-based reactors |
 |
 |
9 |  | 
Which equation does not represent a redox reaction? |
|  | A) | CH4(g) + 2 O2(g) ----> CO2(g) + 2 H2O (l) |
|  | B) | 4 Al4(s) + 3 O2(g) ----> 6 H2O (l) + 4 Al(OH)3(s) |
|  | C) | Zn (s) + 2 MnO2(s) + H2O (l) ----> Zn(OH)2(s) + Mn2O3(s) |
|  | D) | HCl (aq) + NaOH (aq) ----> NaCl (aq) + H2O (l) |
 |
 |
10 |  | 
What type of electrochemical device is shown in this diagram?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A10.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | It is a device for reacting hydrogen with oxygen to produce heat energy. |
|  | B) | It is a fuel cell used in powering earlier missions to outer space. |
|  | C) | It is an alkaline cell such as used in flashlights. |
|  | D) | It is a hybrid cell utilizing a polymer membrane that is permeable to H+ ions. |
 |
 |
11 |  | 
What is the purpose of the "Million Roofs" initiative? |
|  | A) | The purpose is to bring electrochemical cells and batteries to remote areas of the world that are not served by electricity at this time. |
|  | B) | The purpose is to encourage solar cell installation on the roofs of cars, helping to power these cars. |
|  | C) | The purpose is to use solar energy for generation of hydrogen, which can then be used to power fuel cells. |
|  | D) | The purpose is to install arrays of solar cells on rooftops to help meet electricity needs in rural and remote areas of the U.S. and Europe. |
 |
 |
12 |  | 
When compared to using conventional methods, which factors are advantages for using photovoltaic cells to convert sunlight directly into electricity?
I non-polluting in operation
II low maintenance during operation
III modular, so any size PV array can be constructed
IV large amount of space needed for a photovoltaic array
V quiet while in operation
VI high cost of producing the PV cells |
|  | A) | I, II, IV, and V |
|  | B) | I, II, III, and V |
|  | C) | III, IV, and VI |
|  | D) | III, IV, V, and IV |
 |
 |
13 |  | 
What type of semi-conductor is shown in this diagram? Key: The smaller filled circles represent electrons. The larger filled circles represent Si atoms. The patterned circle represents a "doping" atom.
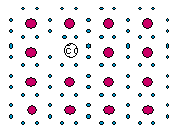 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A13.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8A13.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | a gallium-doped p-type silicon semi-conductor |
|  | B) | a gallium-doped n-type silicon semi-conductor |
|  | C) | an arsenic-doped p-type silicon semi-conductor |
|  | D) | an arsenic-doped n-type silicon semi-conductor |
 |
 |
14 |  | 
Which illustrates the D+T reaction that is widely used in fusion research? |
|  | A) | 21H + 21H ----> 42He |
|  | B) | 31H + 31H ----> 42He + 2 10n |
|  | C) | 63Li + 10n ----> 31H + 42He |
|  | D) | 21H + 31H ----> 42He + 2 10n |
 |
 |
15 |  | 
Which statement is false concerning this reaction of hydrogen:
2 H2(g) + O2(g) ----> 2 H2O (l) [-572 kJ] |
|  | A) | One mole of hydrogen in the reaction would yield -286 kJ of heat. |
|  | B) | The reactants are gases while the product is a liquid (and heat.) |
|  | C) | The formation of hydrogen and oxygen from water in the reverse reaction would require 572 kJ of energy to be input into the reaction. |
|  | D) | None of these statements are false; all are true. |
 |
 |
16 |  | 
Which is not a property of all batteries? |
|  | A) | A battery is specific; and reactions occur at both the anode and cathode. |
|  | B) | A battery has a characteristic electrical potential or voltage. |
|  | C) | A battery has a certain amount or current of electricity that is related to its size. |
|  | D) | A battery has the ability to be recharged. |
 |
 |
17 |  | 
Which is false concerning the properties of hydrogen, particularly concerning its use as an alternative fuel? |
|  | A) | Hydrogen has a high energy density (kJ/g). |
|  | B) | There is virtually no hydrogen present in the earth's atmosphere because large quantities are consumed by the sun. |
|  | C) | It takes a significant amount of energy to separate hydrogen from water. |
|  | D) | There are abundant natural sources of hydrogen in forms other than H2(g). |
 |
 |
18 |  | 
Which statement is false in regards to photovoltaic (solar) cells? |
|  | A) | A photovoltaic cell contains an n-type semiconductor. |
|  | B) | A photovoltaic cell is a device meant to store electricity; its use is growing rapidly. |
|  | C) | A photovoltaic cell converts light from the sun into electricity by a direct route. |
|  | D) | None of these statements are true; all are false. |
 |

