 |
1 |  | 
What equation does not represent a redox reaction? |
|  | A) | 2 AgBr (s) + light ----> 2 Ag (s) + Br2(l) |
|  | B) | 4 Al (s) + 3 O2(g) + 6 H2O (l) ----> 4 Al(OH)3(s) |
|  | C) | Zn (s) + 2 MgO2(s) + H2O (l) ----> Zn(OH)2(s) + Mn2O3(s) |
|  | D) | AgBr (s) + 2 S2O32-(aq) ----> Ag(S2O3)23-(aq) + Br-(aq) |
 |
 |
2 |  | 
This is a schematic of a typical "dry" cell. What is the half-reaction of the reduction in this cell?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | Zn (s) + 2 OH- ----> Zn(OH)2(s) + 2 e- |
|  | B) | Zn (s) + 2 MnO2(s) + 2 e- ----> Mn2O3(s) + 2 OH-(aq) |
|  | C) | Zn (s) + H2O (l) + 2 MnO2(s) ----> Mn2O3(s) + Zn(OH)2(s) |
|  | D) | 2 MnO2(s) + H2O (l) 2 e- ----> Mn2O3(s) + 2 OH-(aq) |
 |
 |
3 |  | 
What does this GM-EV1 all-electric vehicle use to generate horsepower?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (9.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (9.0K)</a>
|
|  | A) | photovoltaic cells |
|  | B) | fusion-based reactors |
|  | C) | methanol-based fuels |
|  | D) | lead-acid storage batteries |
 |
 |
4 |  | 
What type of electrochemical device is shown in this diagram?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (12.0K)</a>
|
|  | A) | It is a device capable of converting chemical energy directly to electricity without any combustion taking place. |
|  | B) | It is an energy-saving device for separating hydrogen from water. |
|  | C) | It is a rechargeable alkaline cell that can be grouped with other cells and used to power space flight. |
|  | D) | It is a device for carrying out the combustion of methane with oxygen to produce heat energy. |
 |
 |
5 |  | 
This reaction takes place in a hydrogen-oxygen fuel cell.
2 H2(g) + O2(g) ----> 2 H2O (l)
What takes place at the anode? |
|  | A) | oxidation of O2 |
|  | B) | oxidation of H2 |
|  | C) | reduction of O2 |
|  | D) | reduction of H2 |
 |
 |
6 |  | 
The term "hybrid car" is most commonly used for a car containing |
|  | A) | two different types of batteries. |
|  | B) | both a battery and a conventional gasoline-powered engine. |
|  | C) | both a conventional gasoline-powered engine and an ethanol-powered engine. |
|  | D) | a battery and a hydrogen-oxygen fuel cell. |
 |
 |
7 |  | 
What type of semiconductor is shown in this diagram? Key: The smaller filled circles represent electrons. The larger filled circles represent silicon atoms. The checked circle represents a "doping" atom.
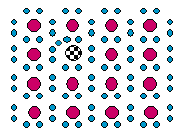 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | an arsenic-doped p-type silicon semiconductor |
|  | B) | an arsenic-doped n-type silicon semiconductor |
|  | C) | a gallium-doped p-type silicon semiconductor |
|  | D) | a gallium-doped n-type silicon semiconductor |
 |
 |
8 |  | 
Arrays of photovoltaic cells are |
|  | A) | in use in many countries, including the U.S., to produce electricity. |
|  | B) | essential for space applications, but cannot be used on the surface of the earth. |
|  | C) | an idea only for the future because of the costs of producing the cells. |
|  | D) | an old technology that has proven to be impractical. |
 |
 |
9 |  | 
Tritium, 31H, is formed by bombarding a Li-6 nucleus with a neutron. What is the other product of this transmutation? |
|  | A) | Li-9, 93Li |
|  | B) | deuterium, 21H |
|  | C) | a beta particle, 0-1e |
|  | D) | an alpha particle, 42He |
 |
 |
10 |  | 
One of the most difficult problems in controlling a deuterium-tritium (D-T) fusion reaction is that of containment of the hot plasma. Which statement describes the first step in the process of inertial confinement? |
|  | A) | Tiny glass spheres containing D-T mixtures are subjected to ultraviolet radiation generated by high-energy lasers. |
|  | B) | D-T mixtures are subjected to strong magnetic and electrical fields, preventing the fusing mixture from touching the walls of the container. |
|  | C) | Very high temperatures and pressures confine the fusing D-T mixture in high-quality surgical steel containers. |
|  | D) | PEM membranes are able to allow the transmission of fusing D-T mixtures long enough for the reaction to take place. |
 |
 |
11 |  | 
Consider this reaction that takes place in a cell.
Zn (s) + 2 MnO2(s) + H2O (l) ----> Zn(OH)2(s) + Mn2O3(s)
Which statement is true about this equation? |
|  | A) | It represents only the half-reaction of oxidation. |
|  | B) | It represents only the half-reaction of reduction. |
|  | C) | It represents the overall reaction that takes place. |
|  | D) | It represents a reaction that is not a redox reaction. |
 |
 |
12 |  | 
The Toyota Prius hybrid car uses |
|  | A) | both a nickel-metal hydride battery and a conventional gasoline-powered engine. |
|  | B) | two different types of electrochemical cells, or batteries. |
|  | C) | a conventional gasoline-powered engine and an ethanol-powered engine. |
|  | D) | an electrochemical cell and a hydrogen-oxygen fuel cell. |
 |
 |
13 |  | 
This type of alkaline cell is commonly used to power flashlights and other similar objects. Which is the anode of the cell?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51622/8B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a>
|
|  | A) | carbon rod |
|  | B) | paste of KOH, MnO2 |
|  | C) | zinc can |
|  | D) | water |
 |
 |
14 |  | 
A device in which the energy created by a spontaneous chemical reaction is converted into electrical energy is |
|  | A) | an electrochemical cell |
|  | B) | an electrolytic cell |
|  | C) | a solar cell |
|  | D) | a hydroelectric cell |
 |
 |
15 |  | 
Consider an ordinary dry-cell flashlight battery: which is not a property of this battery? |
|  | A) | The amount of electricity (voltage) increases with the size of the battery. |
|  | B) | Two specific and different reactions occur: one at the anode and one at the cathode. |
|  | C) | It has a characteristic electrical potential. |
|  | D) | It is irreversible and cannot be recharged. |
 |
 |
16 |  | 
Which has not been implemented in automobiles? |
|  | A) | batteries that power electric motors in electric automobiles |
|  | B) | an internal combustion engine fueled by propane gas |
|  | C) | solar energy for the purpose of recharging the batteries of an electric car |
|  | D) | fusion cells that combine hydrogen isotopes in order to form helium |
 |
 |
17 |  | 
Which is false concerning nuclear fusion? |
|  | A) | Nuclear fusion provides energy for the Sun. |
|  | B) | Nuclear fusion provides massive amounts of energy and no radioactive products. |
|  | C) | Nuclear fusion requires special containment and extremely high heat. |
|  | D) | None of these statements are false; all are true. |
 |
 |
18 |  | 
A device in which electrical energy is used to perform a chemical transformation is called |
|  | A) | an electrochemical cell. |
|  | B) | an electrolytic cell. |
|  | C) | a hydroelectric cell. |
|  | D) | a hydrogen cell. |
 |

