 |
1 |  | 
How many carbon atoms and how many hydrogen atoms are represented by this structure?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 2 C, 3 H |
|  | B) | 6 C, 8 H |
|  | C) | 8 C, 9 H |
|  | D) | 8 C, 8 H |
 |
 |
2 |  | 
Which of these four of the "Big Six" polymers is produced from this monomer by addition polymerization?
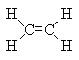 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9AX.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9AX.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | PS; polystyrene |
|  | B) | PVC; polyvinyl chloride |
|  | C) | HDPE; high density polyethylene |
|  | D) | PET; polyethylene terephthalate |
 |
 |
3 |  | 
What is a "buckminsterfullere"? |
|  | A) | It is the trade name of a special type of plastic used in sports equipment. |
|  | B) | It is an allotrope of carbon with molecular formula C60. Its structure resembles the shape of a soccer ball. |
|  | C) | It, like diamond and graphite, is an isotope of carbon and is highly prized for its usefulness in producing plastics. |
|  | D) | It is a product that will aid in the biodegradation of plastics, assuming that appropriate bacteria are added. |
 |
 |
4 |  | 
Addition polymerization is often catalyzed by a chemical free radical. What is a "free radical"? |
|  | A) | It is a chemical species with one or more unpaired electrons. |
|  | B) | It is a chemical species with one or more double bonds. |
|  | C) | It is a chemical species with all electrons paired. |
|  | D) | It is a chemical species with all single bonds. |
 |
 |
5 |  | 
Polyvinyl chloride, PVC, can exhibit many different properties. Which arrangement shows the "head-to-head, tail-to-tail" orientation? |
|  | A) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 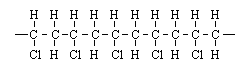 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A5_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
6 |  | 
TeflonÒ is formed by addition polymerization from the monomer shown here. If, on average, about 10,000 monomer units join to form the polymer, what is the average molar mass of the TeflonÒ polymer?
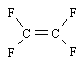 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 0.00100 g/mol |
|  | B) | 100 g/mol |
|  | C) | 10,000 g/mol |
|  | D) | 1,000,000 g/mol |
 |
 |
7 |  | 
Polyproplyene is one of the top six polymers. It is formed from the propylene monomer shown here. Which statement about polypropylene is true?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | Polypropylene is formed by condensation polymerization. The largest percentage of it is used for indoor/outdoor carpeting. |
|  | B) | Polypropylene is formed by addition polymerization. The largest percentage is used for building and construction. |
|  | C) | Polypropylene is formed by addition polymerization. The largest percentage of it is used for packaging. |
|  | D) | Polypropylene is formed by condensation polymerization. The largest percentage of it is used for wall coverings, upholstery, and shower curtains. |
 |
 |
8 |  | 
Both bottles are made of polyethylene. How do they differ?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (6.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A8.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (6.0K)</a>
|
|  | A) | The bottle on the right is made of flexible, low-density, linear polyethylene. The one on the left is made of rigid, high-density, branched polyethylene. |
|  | B) | The bottle on the right is made of flexible, high-density, branched polyethylene. The one of the left is made of rigid, low-density, linear polyethylene. |
|  | C) | The bottle on the right is made of flexible, low-density, branched polyethylene. The one on the left is made of rigid, high-density, linear polyethylene. |
|  | D) | The bottle on the right is made of flexible, high-density linear polyethylene. The one on the left is made of rigid, low-density, branched polyethylene. |
 |
 |
9 |  | 
What is the molar mass for this monomer?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A9.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A9.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 74 g/mol |
|  | B) | 188 g/mol |
|  | C) | 192 g/mol |
|  | D) | 216 g/mol |
 |
 |
10 |  | 
One of the headings in this chapter is "Plastics: Where from and Where to?" Which statement helps to answer that question? |
|  | A) | More than 50% of a barrel of crude oil is used for the production of plastics. About 75% of solid waste is plastics. |
|  | B) | About 20% of a barrel of crude oil is used for the production of plastics. About 15% of all plastics are incinerated. |
|  | C) | About 10% of a barrel of crude oil is used for the production of plastics. About 10% of all plastics are recycled. |
|  | D) | Less than 3% of a barrel of crude oil is used for the production of plastics. About 9% of municipal waste is plastics. |
 |
 |
11 |  | 
KevlarÒ is a type of polymer with great tear resistance. It is used in making bulletproof vests and radial tires, among other applications. These are two monomers used to make KevlarÒ.
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A11.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A11.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (2.0K)</a>
When these two monomers join |
|  | A) | a molecule of CO2 will be eliminated, and an ester linkage will form. |
|  | B) | nothing will be eliminated, and the two monomers will be linked using a hydrogen-to-hydrogen bond. |
|  | C) | a molecule of H2O will be eliminated, and an amide (peptide) linkage will form. |
|  | D) | nothing will be eliminated, and the two monomers will be linked using a carbon-to-carbon bond. |
 |
 |
12 |  | 
DacronÒ is a popular synthetic fabric. The linkage in this polymer is shown in this structure. What is the name of this class of polymer?
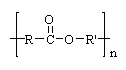 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A12.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A12.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | polyamide |
|  | B) | polyethylene |
|  | C) | polyester |
|  | D) | polyglycol |
 |
 |
13 |  | 
This is one of the coding symbols adopted by the plastics industry for packaging materials. What is the purpose of these coding symbols?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A13.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A13.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | to make recycling easier by making the identification of the plastics easier |
|  | B) | to help manufacturers identify the safety restrictions for the use of each type of plastic |
|  | C) | to facilitate identification for the monomers needed to produce the polymer indicated |
|  | D) | to create a code that will show how the polymer ranks in tons produced per year in the United States |
 |
 |
14 |  | 
Condensation reactions are reactions in which two molecules combine (generally) by producing a water molecule. Which incorrectly pairs a natural or man-made polymer with the type of condensation bond that it forms? |
|  | A) | polyethylene terphthalate, ester |
|  | B) | nylon, amide (peptide) |
|  | C) | protein, amide (peptide) |
|  | D) | polyethylene, ester |
 |
 |
15 |  | 
Which represents a mechanism or method by which polymers can be degraded? |
|  | A) | a special PET structure, Biomax, that is biodegradable |
|  | B) | bacterial enzymes |
|  | C) | bond breaking by UV light |
|  | D) | All three represent mechanisms or methods by which polymers can be degraded. |
 |
 |
16 |  | 
The issue of using paper or plastic bags arises at a grocery store. Which is false concerning the advantages and disadvantages of paper versus plastic? |
|  | A) | Plastic bags cost more than paper bags. |
|  | B) | Plastics bags occupy less space than paper bags. |
|  | C) | Paper is only marginally more degradable than plastic. |
|  | D) | All three of these statements are false. |
 |

