 |
1 |  | 
All plastics are made up of long chains of atoms that are held together with |
|  | A) | hydrogen bonds. |
|  | B) | ionic bonds. |
|  | C) | covalent bonds. |
|  | D) | peptide bonds. |
 |
 |
2 |  | 
This diagram indicates the arrangement of polyethylene chains in a necked region formed by stretching a polyethylene plastic strip. Which statement best describes what is happening at the molecular level as the strip narrows and "necks down"?
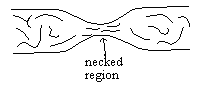 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | The previously mixed-up molecular chains move. They shift, slide, and become aligned parallel to each other. |
|  | B) | Cross-linking takes place in the necked area, now that there is increased opportunity for additional polymerization to take place. |
|  | C) | Several new branches form in the necked area, leading to a relatively low-density polyethylene in this region. |
|  | D) | Several changes take place in the chemical nature of the polymer chain, caused by the closeness of the previously dispersed chains. |
 |
 |
3 |  | 
Many popular synthetic fabrics are condensation polymers with this linkage. What is the name of this linkage?
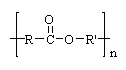 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A12.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A12.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | polyethylene |
|  | B) | polyester |
|  | C) | polycarbonate |
|  | D) | polypeptide |
 |
 |
4 |  | 
Two apparently identical black balls were dropped on a table. They were made of the same polymer. One bounced, the other did not. What can you conclude about the non-bouncing "sad" ball relative to the bouncing "happy" ball?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | The form of the polymer used in the "sad" ball is likely to be highly cross-linked but the form of the polymer used in the "happy" ball contains fewer cross-links. |
|  | B) | The form of the polymer used in the "sad" ball is likely to be completely linear but the form of the polymer used in the "happy" ball is branched. |
|  | C) | The form of the polymer used in the "sad" ball is likely to have a lower average molecular mass than the form of the polymer used in the "happy" ball. |
|  | D) | The form of the polymer used in the "sad" ball is likely to have been formed with the use of plasticizers, but the form of the polymer used in the "happy" ball does not contain any plasticizers. |
 |
 |
5 |  | 
Every year, about 250 pounds of plastics are produced for each man, woman, and child in the U.S. Most of this material currently finds its way into landfills. Which are alternative strategies for lessening the environmental impact of plastics?
I incineration
II biodegradation
III recycling
IV source reduction |
|  | A) | I, II, and III only |
|  | B) | I, III, and IV only |
|  | C) | I, II, and IV only |
|  | D) | I, II, III, and IV |
 |
 |
6 |  | 
Soft contact lenses are made from |
|  | A) | polymethylmethacrylate, PPMA, with silicone fluoropolymers |
|  | B) | hydrogel hydroxyethylmethacrylate, HEMA |
|  | C) | rigid gas permeable polymers, RGP |
|  | D) | fluorosilicone acrylate, FSA |
 |
 |
7 |  | 
Kevlarâ is a type of nylon called an aramid that contains aromatic rings. Because of its great mechanical strength, Kevlarâ is used in bulletproof clothing and in radial tires. The two monomers for producing Kevlarâ are these.
Monomer 1
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
Monomer 2
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
When these two monomers join, which linkage between monomers forms the resulting polymer? |
|  | A) | 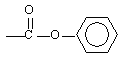 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 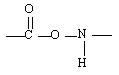 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_5.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B7_6.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
8 |  | 
This is the structure for the styrene monomer used to make polystyrene and Styrofoamâ. What is the chemical formula for the styrene monomer?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | C2H3 |
|  | B) | C8H3 |
|  | C) | C8H8 |
|  | D) | C8H9 |
 |
 |
9 |  | 
Which of the "Big Six" polymers is not formed by addition polymerization? |
|  | A) | PVC; polyvinyl chloride |
|  | B) | PS; polystyrene |
|  | C) | PET; polyethylene terephthalate |
|  | D) | LDPE; low density polyethylene |
 |
 |
10 |  | 
Which monomer is the starting material for the most commonly produced polymer in the United States? |
|  | A) | 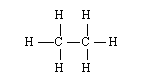 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) | 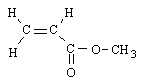 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B10_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
11 |  | 
Over 4.1 billion pounds of high-grade polyethylene terephthalate were produced in 1997, and close to five billion pounds in 2000. What is the major use of this polymer? |
|  | A) | building material |
|  | B) | car bodies |
|  | C) | electrical insulation |
|  | D) | plastic bottles |
 |
 |
12 |  | 
Polypropylene is one of the top six polymers. It is formed from the propylene monomer show here. Which choice shows the formation of the polymer?
 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9A7.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a>
|
|  | A) | 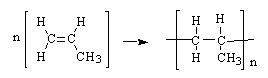 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_1.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_2.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 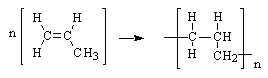 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_3.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) |  <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0072410159/51623/9B12_4.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
 |
 |
13 |  | 
Styrofoamâ cups dissolve in benzene, C6H6, but do not dissolve in water. Which statement best describes the reasons behind these observations? |
|  | A) | Styrofoamâ is a polar polymer and therefore dissolves in polar benzene, but cannot dissolve in nonpolar water. |
|  | B) | Styrofoamâ is a nonpolar polymer and therefore can dissolve in nonpolar benzene, but cannot dissolve in polar water. |
|  | C) | Styrofoamâ is a polar polymer and therefore dissolves in nonpolar benzene, but cannot dissolve in polar water. |
|  | D) | Styrofoamâ is a nonpolar polymer and therefore can dissolve in polar benzene, but cannot dissolve in nonpolar water. |
 |
 |
14 |  | 
Which is not a molecular, structural variable responsible for polymer properties? |
|  | A) | the 3-D arrangement of monomer units |
|  | B) | addition versus condensation polymerization mechanisms |
|  | C) | branching of the chain |
|  | D) | orientation of monomers within the chain |
 |
 |
15 |  | 
Which is not a form of elemental carbon? |
|  | A) | methane |
|  | B) | graphite |
|  | C) | diamond |
|  | D) | buckyballs |
 |
 |
16 |  | 
Polyethylene contributes two entries to the list of the "big six" polymers. Which is false concerning these polymers? |
|  | A) | LDPE has branched chains and is used in grocery bags. |
|  | B) | HDPE has straight chains and is used in toys. |
|  | C) | HDPE is a condensation polymer; LDPE is an addition polymer. |
|  | D) | HDPE is more rigid and stronger than LDPE; HDPE is composed of many more monomers than LDPE. |
 |

