
(See related pages)
| Clapeyron equation | named after the French engineer and physicist E. Clapeyron (1799-1864), relates the enthalpy change associated with a phase change (such as the enthalpy of vaporization hfg) from knowledge of P, v, and T data alone. |
| Clapeyron-Clausius equation | is used to determine the variation of saturation pressure with temperature. |
| Cyclic relation of partial derivatives | shows that the derivatives of a function of two variables are related in a cyclic manner by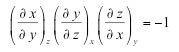 |
| Derivative of a function | f(x) with respect to x represents the rate of change of f with x. The derivative is equivalent to steepness of a curve at a point as measured by the slope of a line tangent to the curve at that point.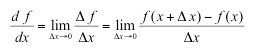 |
| Enthalpy departure | is the difference between the enthalpy of a real gas and the enthalpy of the gas at an ideal gas state and it represents the variation of the enthalpy of a gas with pressure at a fixed temperature. |
| Enthalpy departure factor | is the nondimensionalized form of the enthalpy departure. |
| Entropy departure | is the difference between the entropy of a real gas at a given P and T and the entropy of the gas at an ideal gas state at the same P and T . |
| Entropy departure factor | is the nondimensionalized form of the entropy departure. |
| Generalized enthalpy departure chart | is a plot of the enthalpy departure factor as a function of reduced pressure and reduced temperature. It is used to determine the deviation of the enthalpy of a gas at a given P and T from the enthalpy of an ideal gas at the same T. |
| Generalized entropy departure chart | is a plot of the entropy departure factor as a function of reduced pressure and reduced temperature. It is used to determine the deviation of the entropy of a gas at a given P and T from the entropy of an ideal gas at the same P and T. |
| Gibbs function | g is defined as g = h - Ts. |
| Helmholtz function | a is defined as a = u - Ts. |
| Inversion line | is the line that passes through the points of zero slope of constant-enthalpy lines or zero Joule-Thomson coefficient on the T-P diagram. The slopes of the h = constant lines are negative (μJT < 0) at states to the right of the inversion line and positive (μJT > 0) to the left of the inversion line. |
| Inversion temperature | is the temperature at a point where a constant-enthalpy line intersects the inversion line. |
| Isothermal compressibility | relates how volume changes when pressure changes as temperature is held constant. |
| Joule-Thomson coefficient | μJT is a measure of the change in temperature with pressure during a constant-enthalpy process. |
| Maximum inversion temperature | is the temperature at the intersection of the P= 0 line (ordinate) on the T-P diagram and the upper part of the inversion line. |
| Maxwell relations | are equations that relate the partial derivatives of properties P, v, T, and s of a simple compressible system to each other. |
| Mayer relation | named in honor of the German physician and physicist J. R. Mayer (1814-1878, shows how the difference between the constant-pressure specific heat and constant-volume specific heat is related to the specific volume, temperature, isothermal compressibility, and volume expansivity. |
| Partial derivative | is the change in a function that depends on two (or more) variables, such as z = z (x, y), when allowing one variable to change while holding the others constant and observing the change in the function as another variable is held constant. The variation of z(x, y) with x when y is held constant is called the partial derivative of z with respect to x.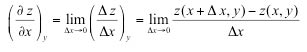 |
| Reciprocity relation | shows that the inverse of a partial derivative is equal to its reciprocal.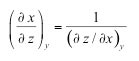 |
| Total differential | of a dependent variable in terms of its partial derivatives with respect to the independent variables is expressed as, for z = z (x, y), |
| Volume expansivity | (also called the coefficient of volumetric expansion) relates how volume changes when temperature changes when pressure is held constant. |