 |
| 1 |  | 
What is
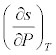 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240306/formula21.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> for an ideal gas? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240306/formula21.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (10.0K)</a> for an ideal gas? |
|  | A) | - 1 / P |
|  | B) | - R / P |
|  | C) | - 1 / T |
|  | D) | - R / T |
|
|
 |
| 2 |  | 
The slope of the R-134a liquid-vapor saturation line at 0°C on a phase diagram is: |
|  | A) | 10.6 kPa/K |
|  | B) | 10.6 K/kPa |
|  | C) | 15.3 kPa/K |
|  | D) | 15.3 K/kPa |
|
|
 |
| 3 |  | 
According to the Gibbs relations, what is the relationship between the enthalpy of vaporization and entropy of vaporization? |
|  | A) | sfg = ufg / T |
|  | B) | sfg = ufg / P |
|  | C) | sfg = hfg / T |
|  | D) | sfg = hfg / P |
|
|
 |
| 4 |  | 
What is 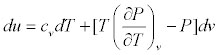 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240306/formula22.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> for an ideal gas? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=jpg::::/sites/dl/free/0073398179/240306/formula22.jpg','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (13.0K)</a> for an ideal gas? |
|  | A) | du = cvdT - v / R |
|  | B) | du = cvdT |
|  | C) | du = cvdT - T / P |
|  | D) | none of these |
|
|
 |
| 5 |  | 
The entropy change of an ideal gas whose specific heats are constant is given by: |
|  | A) | cPln (T2 / T1) - R ln (P2 / P1) |
|  | B) | cPln (P2 / P1) - R ln (T2 / T1) |
|  | C) | cvln (T2 - T1) - R ln (P2 / P1) |
|  | D) | cvln (T2T2 - T1) - R ln (P2 / P1) |
|
|
 |
| 6 |  | 
Working fluids used in refrigeration cycles decrease in temperature as they pass through the throttle valve. The Joule-Thompson coefficient of these fluids is: |
|  | A) | Negative |
|  | B) | 0 |
|  | C) | Positive |
|  | D) | None of these |
|
|
 |
| 7 |  | 
The constant pressure specific heat of an ideal gas is given by cP = a + bT + cT 2, where a, b, and c are constants and T is the absolute temperature. The entropy change of this gas is given by: |
|  | A) | a (T2–T1) –R ln (P2 / P1) |
|  | B) | a ln (T2/ T1) + b (T2–T1) –R ln (P2/ P1) |
|  | C) | a ln (T2/ T1) + b (T2–T1) + c (T22–T12) / 2 –R ln (P2/ P1) |
|  | D) | a (T2–T1) + b (T22–T12) / 2 + c (T23–T13) / 3 –R ln (P2/ P1) |
|
|
 |
| 8 |  | 
What is the enthalpy departure of carbon dioxide at 14.8 MPa, 456 K? |
|  | A) | 0.8 |
|  | B) | 1.0 |
|  | C) | 1.5 |
|  | D) | 2.0 |
|
|
 |
| 9 |  | 
What is the difference in the enthalpy of carbon dioxide at state 1 where P1 = 14.8 MPa, T1 = 460 K and state 2 where P2 = 100 kPa, T2 = 300 K? |
|  | A) | -3960 kJ/k mol |
|  | B) | -5240 kJ/k mol |
|  | C) | -6490 kJ/k mol |
|  | D) | -7320 kJ/k mol |
|
|
 |
| 10 |  | 
What is the difference in the entropy of carbon dioxide at state 1 where P1 = 14.8 MPa, T1 = 460 K and state 2 where P2 = 100 kPa, T2 = 300 K? |
|  | A) | -5.32 kJ/kg-mol-K |
|  | B) | 0 kJ/kg-mol-K |
|  | C) | 14.37 kJ/kg-mol-K |
|  | D) | 28.48 kJ/kg-mol-K |
|
|

