Please note that this quiz uses special characters. Not all browsers display special characters properly. For best results please use Internet Explorer 5.5 or newer or Netscape 6.2 or newer.
 |
| 1 |  | 
An alkaline earth element is radioactive. It and its daughter elements decay by emitting a total of three alpha particles in succession. In what group of the periodic table is the element resulting from the emission of the third alpha particle? |
|  | A) | 4A (14) |
|  | B) | 5A (15) |
|  | C) | 6A (16) |
|  | D) | 7A (17) |
|  | E) | 8A (18) |
|
|
 |
| 2 |  | 
Given that the stable isotopes of potassium are potassium-39 and potassium-41, what type of emission should be expected from the radioisotope, potassium-38? |
|  | A) | proton |
|  | B) | neutron |
|  | C) | β particle |
|  | D) | positron |
|  | E) | a particle |
|
|
 |
| 3 |  | 
Which one of the following isotopes is definitely unstable? |
|  | A) | 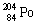 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image19.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image19.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | B) | 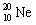 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image20.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image20.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | C) | 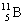 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image21.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image21.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | D) | 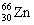 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image22.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image22.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|  | E) | 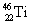 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image23.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image23.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|
|
 |
| 4 |  | 
Alpha decay is most common for |
|  | A) | light, neutron-rich nuclei. |
|  | B) | light, neutron-poor nuclei. |
|  | C) | light nuclei which lie close to the band of stability. |
|  | D) | nuclei with an unfavorable N/Z ratio. |
|  | E) | heavy nuclei, beyond the end of the band of stability. |
|
|
 |
| 5 |  | 
The half-life of 129I is 1.72 ´ 107 years. Consider a sample that contains 2.39 ´ 106 atoms of this isotope. How many of those atoms will have disintegrated after 3.44 ´ 107 years? |
|  | A) | 7.97 ´ 105 |
|  | B) | 5.97 ´ 105 |
|  | C) | 1.19 ´ 106 |
|  | D) | 1.79 ´ 106 |
|  | E) | 2.39 ´ 105 |
|
|
 |
| 6 |  | 
Cesium-134 is a b emitter with a half-life of 2.0 years. How much of a 2.50 microgram sample of cesium-134 will remain after 10.0 years? |
|  | A) | 0.500#m g |
|  | B) | 0.250#m g |
|  | C) | 0.0781#m g |
|  | D) | 0.00244#m g |
|  | E) | None of the above |
|
|
 |
| 7 |  | 
The specific activity resulting from the decay of carbon-14 in a fresh wood sample is 15.2 disintegrations per minute per gram of carbon. A piece of charcoal from an archeological site has a specific activity of 2.93 disintegrations per minute per gram of carbon. Given that the half-life of carbon-14 is 5730 years, calculate or estimate the "age" of the charcoal sample. |
|  | A) | 1.10 ´ 103 years |
|  | B) | 8.96 ´ 103 years |
|  | C) | 1.36 ´ 104 years |
|  | D) | 1.92 ´ 104 years |
|  | E) | 2.97 ´ 104 years |
|
|
 |
| 8 |  | 
What symbol is needed to complete the nuclear equation: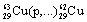 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image24.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> ? <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image24.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> ? |
|  | A) | b |
|  | B) | n |
|  | C) | b+ |
|  | D) | a |
|  | E) | 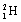 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image25.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif::::/sites/dl/free/0073402699/37547/Image25.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|
|
|
 |
| 9 |  | 
Which one of the following statements is incorrect? |
|  | A) | Alpha particles emitted by a source outside of the human body do not affect internal organs. |
|  | B) | Beta particles cannot penetrate ordinary light clothing. |
|  | C) | Gamma rays can penetrate deeply into living tissue. |
|  | D) | Alpha particles emitted by a source inside the human body can cause significant damage to internal organs. |
|  | E) | Positrons can penetrate ordinary light clothing. |
|
|
 |
| 10 |  | 
The difference between the rad and the rem is |
|  | A) | the rem is a rad per year. |
|  | B) | the rad takes into account the type of radiation. |
|  | C) | the rem takes into account the effect on the particular biological tissue. |
|  | D) | the rem is a rad per kilogram. |
|  | E) | none of the above. |
|
|
 |
| 11 |  | 
The atomic mass of chromium-52 is 51.940509 amu. Use data from your textbook to calculate the mass defect (Dm) for this isotope. |
|  | A) | 0.486551 amu |
|  | B) | 0.489911 amu |
|  | C) | 0.466391 amu |
|  | D) | 0.510071 amu |
|  | E) | 0.488231 amu |
|
|
 |
| 12 |  | 
Given that the mass defect (Dm) for carbon-12 is 1.648 ´ 10-25 g, what is the average binding energy per nucleon of a 12C nucleus, in J? |
|  | A) | 1.48 ´ 10-11 J |
|  | B) | 2.47 ´ 10-12 J |
|  | C) | 1.23 ´ 10-12 J |
|  | D) | 7.42 ´ 1014 J |
|  | E) | None of the above. |
|
|
 |
| 13 |  | 
How are nuclides with mass numbers above 230 formed? |
|  | A) | by the s-process during the normal lifetimes of stars |
|  | B) | by the s-process during supernova explosions |
|  | C) | by the r-process during the normal lifetimes of stars |
|  | D) | by the r-process during supernova explosions |
|  | E) | by none of the above mechanisms |
|
|
 |
| 14 |  | 
Which of the following factors affects the rate of a fission reaction in a nuclear reactor? |
|  | A) | the temperature in the reactor |
|  | B) | the pressure in the reactor |
|  | C) | the concentration of neutrons in the reactor |
|  | D) | the presence of a catalyst |
|  | E) | none of the above |
|
|
 |
| 15 |  | 
If each step in a fission chain reaction were to produce, on average, 2.5 neutrons, how many neutrons would be released in the 10th step, starting from one neutron? |
|  | A) | 102.5 |
|  | B) | 2.510 |
|  | C) | 2.5 ´ 10 |
|  | D) | 2.5 ´ 210 |
|  | E) | none of the above |
|
|

