 |
1 |  | 
Which one of the following compounds is most likely to be ionic? |
|  | A) | HNF2 |
|  | B) | H2CO |
|  | C) | N2H4 |
|  | D) | CaCl2 |
|  | E) | ICl |
 |
 |
2 |  | 
Based on ionic radii and charges, which of the following solids would have the greatest lattice energy? |
|  | A) | SrO |
|  | B) | CsI |
|  | C) | MgCl2 |
|  | D) | KF |
|  | E) | NaF |
 |
 |
3 |  | 
In Ba(CN)2, the bonding is |
|  | A) | essentially ionic. |
|  | B) | essentially covalent. |
|  | C) | both ionic and covalent. |
|  | D) | neither ionic nor covalent. |
 |
 |
4 |  | 
Which of the following represents the most polar bond? |
|  | A) | B-C |
|  | B) | S-O |
|  | C) | C-O |
|  | D) | B-O |
|  | E) | C-C |
 |
 |
5 |  | 
Which of the Lewis structure(s) is(are) incorrect? |
|  | A) | 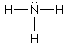 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5a.gif','popWin', 'width=86,height=62,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5a.gif','popWin', 'width=86,height=62,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | B) | 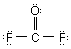 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5b.gif','popWin', 'width=87,height=65,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5b.gif','popWin', 'width=87,height=65,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | C) | 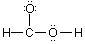 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5c.gif','popWin', 'width=101,height=60,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5c.gif','popWin', 'width=101,height=60,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (1.0K)</a> |
|  | D) | 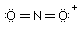 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5d.gif','popWin', 'width=97,height=45,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq5d.gif','popWin', 'width=97,height=45,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | E) | C and D. |
 |
 |
6 |  | 
All of the following molecules contain only single bonds except |
|  | A) | H2O2 |
|  | B) | Cl2 |
|  | C) | CH4 |
|  | D) | H2O |
|  | E) | SO2 |
 |
 |
7 |  | 
The formal charge on the nitrogen atom in the nitrate ion is |
|  | A) | +2 |
|  | B) | +1 |
|  | C) | 0 |
|  | D) | -1 |
|  | E) | -2 |
 |
 |
8 |  | 
The sulfate ion is tetrahedral with four equal S-O distances of 149 pm. Which of the following is the most reasonable structure consistent with these facts? |
|  | A) | 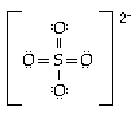 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8a.gif','popWin', 'width=155,height=134,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8a.gif','popWin', 'width=155,height=134,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | B) | 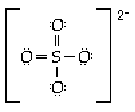 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8b.gif','popWin', 'width=NaN,height=NaN,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | C) | 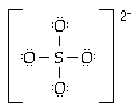 <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8c.gif','popWin', 'width=151,height=128,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> <a onClick="window.open('/olcweb/cgi/pluginpop.cgi?it=gif:: ::/sites/dl/free/0073656011/17477/ch9quizaq8c.gif','popWin', 'width=151,height=128,resizable,scrollbars');" href="#"><img valign="absmiddle" height="16" width="16" border="0" src="/olcweb/styles/shared/linkicons/image.gif"> (0.0K)</a> |
|  | D) | None of the above. |
 |
 |
9 |  | 
In which of the following species does the central atom violate the octet rule? |
|  | A) | CH4 |
|  | B) | SF4 |
|  | C) | PCl4+ |
|  | D) | CCl3+ |
|  | E) | NH3 |
 |
 |
10 |  | 
Estimate the enthalpy change for the reaction C2H4(g) + H2(g) ----> C2H6(g), given the following bond energies: BE(H-H) = 436 kJ/mol; BE(C-H) = 414 kJ/mol; BE(C-C) = 347 kJ/mol; BE(C=C) = 620 kJ/mol. |
|  | A) | -119 kJ |
|  | B) | +119 kJ |
|  | C) | -392 kJ |
|  | D) | +392 kJ |
|  | E) | None of the above. |
 |



 2002 McGraw-Hill Higher Education
2002 McGraw-Hill Higher Education